NF-Y Binds to Both G1- and G2-Specific Cyclin Promoters; a Possible Role in Linking CDK2/Cyclin a to CDK1/Cyclin B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cryoelectron Microscopy Structure of the Human CDK-Activating Kinase
The cryoelectron microscopy structure of the human CDK-activating kinase Basil J. Grebera,b,1,2, Juan M. Perez-Bertoldic, Kif Limd, Anthony T. Iavaronee, Daniel B. Tosoa, and Eva Nogalesa,b,d,f,2 aCalifornia Institute for Quantitative Biosciences (QB3), University of California, Berkeley, CA 94720; bMolecular Biophysics and Integrative Bio-Imaging Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720; cBiophysics Graduate Group, University of California, Berkeley, CA 94720; dDepartment of Molecular and Cell Biology, University of California, Berkeley, CA 94720; eQB3/Chemistry Mass Spectrometry Facility, University of California, Berkeley, CA 94720; and fHoward Hughes Medical Institute, University of California, Berkeley, CA 94720 Edited by Seth A. Darst, Rockefeller University, New York, NY, and approved August 4, 2020 (received for review May 14, 2020) The human CDK-activating kinase (CAK), a complex composed of phosphoryl transfer (11). However, in addition to cyclin binding, cyclin-dependent kinase (CDK) 7, cyclin H, and MAT1, is a critical full activation of cell cycle CDKs requires phosphorylation of the regulator of transcription initiation and the cell cycle. It acts by T-loop (9, 12). In animal cells, these activating phosphorylations phosphorylating the C-terminal heptapeptide repeat domain of are carried out by CDK7 (13, 14), itself a cyclin-dependent ki- the RNA polymerase II (Pol II) subunit RPB1, which is an important nase whose activity depends on cyclin H (14). regulatory event in transcription initiation by Pol II, and it phos- In human and other metazoan cells, regulation of transcription phorylates the regulatory T-loop of CDKs that control cell cycle initiation by phosphorylation of the Pol II-CTD and phosphor- progression. -

Targeting Cyclin-Dependent Kinase 9 and Myeloid Cell Leukaemia 1 in MYC-Driven B-Cell Lymphoma
Targeting cyclin-dependent kinase 9 and myeloid cell leukaemia 1 in MYC-driven B-cell lymphoma Gareth Peter Gregory ORCID ID: 0000-0002-4170-0682 Thesis for Doctor of Philosophy September 2016 Sir Peter MacCallum Department of Oncology The University of Melbourne Doctor of Philosophy Submitted in total fulfilment of the degree of Abstract Aggressive B-cell lymphomas include diffuse large B-cell lymphoma, Burkitt lymphoma and intermediate forms. Despite high response rates to conventional immuno-chemotherapeutic approaches, an unmet need for novel therapeutic by resistance to chemotherapy and radiotherapy. The proto-oncogene MYC is strategies is required in the setting of relapsed and refractory disease, typified frequently dysregulated in the aggressive B-cell lymphomas, however, it has proven an elusive direct therapeutic target. MYC-dysregulated disease maintains a ‘transcriptionally-addicted’ state, whereby perturbation of A significant body of evidence is accumulating to suggest that RNA polymerase II activity may indirectly antagonise MYC activity. Furthermore, very recent studies implicate anti-apoptotic myeloid cell leukaemia 1 (MCL-1) as a critical survival determinant of MYC-driven lymphoma. This thesis utilises pharmacologic and genetic techniques in MYC-driven models of aggressive B-cell lymphoma to demonstrate that cyclin-dependent kinase 9 (CDK9) and MCL-1 are oncogenic dependencies of this subset of disease. The cyclin-dependent kinase inhibitor, dinaciclib, and more selective CDK9 inhibitors downregulation of MCL1 are used -

Datasheet for CDK1-Cyclin B
of T161 is required for activation of the CDK1- Supplied in: 100 mM NaCl, 50 mM HEPES Specific Activity: ~1,000,000 units/mg cyclin B complex and is mediated by the CDK (pH 7.5 @ 25°C), 0.1 mM EDTA, 1 mM DTT, 0.01% CDK1-cyclin B activating kinase (CAK). During G2 phase, Brij 35 and 50% glycerol. Molecular Weight: CDK1 (34 kDa), cyclin B CDK1-cyclin B complex is held in an inactive state (48 kDa). The apparent molecular weight of cyclin by phosphorylation of CDK1 at the two negative Reagents Supplied with Enzyme: B on SDS-PAGE is about 60 kDa. 1-800-632-7799 regulatory sites, T14 and Y15 by CDK1 inhibitory 10X NEBuffer for Protein Kinases (PK). [email protected] protein kinases, Myt1 and Wee1 respectively. Quality Control Assays www.neb.com Dephosphorylation of T14 and Y15 by cell division Reaction Conditions: 1X NEBuffer for PK (NEB Protease Activity: After incubation of 100 units #B6022), supplemented with 200 µM ATP (NEB P6020S 032120413041 cycle 25 (CDC25) protein phosphatase in late G2 of CDK1-cyclin B with a standard mixture phase activates the CDK1-cyclin B complex and #P0756) and gamma-labeled ATP to a final specific of proteins for 2 hours at 30°C, no proteolytic activity of 100–500 µCi/µmol. Incubate at 30°C. triggers the initiation of mitosis. During expression activity could be detected by SDS-PAGE analysis. P6020S in insect cells, the recombiniant CDK1-cyclin B is 1X NEBuffer for PK: Phosphatase Activity: After incubation of 200 units 20,000 U/ml Lot: 0321204 activated in vivo by endogenous kinase (1–4). -
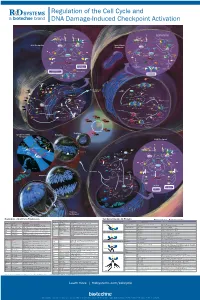
Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation
RnDSy-lu-2945 Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation IR UV IR Stalled Replication Forks/ BRCA1 Rad50 Long Stretches of ss-DNA Rad50 Mre11 BRCA1 Nbs1 Rad9-Rad1-Hus1 Mre11 RPA MDC1 γ-H2AX DNA Pol α/Primase RFC2-5 MDC1 Nbs1 53BP1 MCM2-7 53BP1 γ-H2AX Rad17 Claspin MCM10 Rad9-Rad1-Hus1 TopBP1 CDC45 G1/S Checkpoint Intra-S-Phase RFC2-5 ATM ATR TopBP1 Rad17 ATRIP ATM Checkpoint Claspin Chk2 Chk1 Chk2 Chk1 ATR Rad50 ATRIP Mre11 FANCD2 Ubiquitin MDM2 MDM2 Nbs1 CDC25A Rad50 Mre11 BRCA1 Ub-mediated Phosphatase p53 CDC25A Ubiquitin p53 FANCD2 Phosphatase Degradation Nbs1 p53 p53 CDK2 p21 p21 BRCA1 Ub-mediated SMC1 Degradation Cyclin E/A SMC1 CDK2 Slow S Phase CDC45 Progression p21 DNA Pol α/Primase Slow S Phase p21 Cyclin E Progression Maintenance of Inhibition of New CDC6 CDT1 CDC45 G1/S Arrest Origin Firing ORC MCM2-7 MCM2-7 Recovery of Stalled Replication Forks Inhibition of MCM10 MCM10 Replication Origin Firing DNA Pol α/Primase ORI CDC6 CDT1 MCM2-7 ORC S Phase Delay MCM2-7 MCM10 MCM10 ORI Geminin EGF EGF R GAB-1 CDC6 CDT1 ORC MCM2-7 PI 3-Kinase p70 S6K MCM2-7 S6 Protein Translation Pre-RC (G1) GAB-2 MCM10 GSK-3 TSC1/2 MCM10 ORI PIP2 TOR Promotes Replication CAK EGF Origin Firing Origin PIP3 Activation CDK2 EGF R Akt CDC25A PDK-1 Phosphatase Cyclin E/A SHIP CIP/KIP (p21, p27, p57) (Active) PLCγ PP2A (Active) PTEN CDC45 PIP2 CAK Unwinding RPA CDC7 CDK2 IP3 DAG (Active) Positive DBF4 α Feedback CDC25A DNA Pol /Primase Cyclin E Loop Phosphatase PKC ORC RAS CDK4/6 CDK2 (Active) Cyclin E MCM10 CDC45 RPA IP Receptor -

Human P14arf-Mediated Cell Cycle Arrest Strictly Depends on Intact P53 Signaling Pathways
Oncogene (2002) 21, 3207 ± 3212 ã 2002 Nature Publishing Group All rights reserved 0950 ± 9232/02 $25.00 www.nature.com/onc Human p14ARF-mediated cell cycle arrest strictly depends on intact p53 signaling pathways H Oliver Weber1,2, Temesgen Samuel1,3, Pia Rauch1 and Jens Oliver Funk*,1 1Laboratory of Molecular Tumor Biology, Department of Dermatology, University of Erlangen-Nuremberg, 91052 Erlangen, Germany; 2Regulation of Cell Growth Laboratory, National Cancer Institute, Frederick, Maryland, MD 21702-1201, USA The tumor suppressor ARF is transcribed from the INK4a/ 4 and 6 activities, thus leading to decreased phosphor- ARF locus in partly overlapping reading frames with the ylation of RB and to G1 arrest. Cells that are de®cient CDK inhibitor p16Ink4a. ARF is able to antagonize the for RB are resistant to p16Ink4a-mediated cell cycle MDM2-mediated ubiquitination and degradation of p53, arrest (Sherr and Roberts, 1995; Weinberg, 1995). leading to either cell cycle arrest or apoptosis, depending on ARF is also a cell cyle inhibitor. It does not directly the cellular context. However, recent data point to inhibit CDKs but interferes with the function of additional p53-independent functions of mouse p19ARF. MDM2 to destabilize p53. ARF may be activated by Little is known about the dependency of human p14ARF aberrant activation of oncoproteins such as Ras function on p53 and its downstream genes. Therefore, we (Palmero et al., 1998), c-myc (Zindy et al., 1998), analysed the mechanism of p14ARF-induced cell cycle arrest E1A (de Stanchina et al., 1998), Abl (Radfar et al., in several human cell types. -

21-Containing C.Yclin Kinases Exist in Oth Acnve and Lnacnve States
Downloaded from genesdev.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press 21-containing c.yclin kinases exist in oth acnve and lnacnve states Hui Zhang, Gregory J. Hannon, and David Beach Howard Hughes Medical Institute, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724 USA In normal fibroblasts CDKs exist predominantly in p21/PCNA/cyclin/CDK quaternary complexes, whereas in p53-deficient cells, p21 expression is depressed and the kinases are reduced to a cyclin/CDK binary state, p21 is a universal cyclin kinase inhibitor, but we show here that p21-containing complexes exist in both catalytically active and inactive forms. This finding challenges the current view that active cyclin kinases function only in the binary state and reveals the subtlety with which tumor-suppressor proteins modulate the cell cycle. [Key Words: p21-containing cyclin kinase; cell cycle progression; human fibroblasts; quaternary complex; kinase inhibitor] Received May 12, 1994; revised version accepted June 16, 1994. Much of our current understanding of the regulation of their relative affinities vary with each enzyme (Gu et al. the cell division cycle has emerged from studies of a 1993; Harper et al. 1993; Xiong et al. 1993b). Further- family of protein kinases [cdc2, CDC28, and generically more, several lines of evidence suggest that p21 expres- cyclin-dependent kinase (CDK)] and their inhibitors and sion is regulated by the p53 tumor-suppressor protein activators (for review, see Sherr 1993). A critical step in (E1-Deiry et al. 1993; Xiong et al. 1993b). Thus, cells understanding cell cycle control was the discovery that derived from certain p53-deficient Li-Fraumeni patients CDKs interact with cyclins, proteins that serve as essen- lack p21 associated with cyclin kinases (Xiong et al. -

Cytometry of Cyclin Proteins
Reprinted with permission of Cytometry Part A, John Wiley and Sons, Inc. Cytometry of Cyclin Proteins Zbigniew Darzynkiewicz, Jianping Gong, Gloria Juan, Barbara Ardelt, and Frank Traganos The Cancer Research Institute, New York Medical College, Valhalla, New York Received for publication January 22, 1996; accepted March 11, 1996 Cyclins are key components of the cell cycle pro- gests that the partner kinase CDK4 (which upon ac- gression machinery. They activate their partner cy- tivation by D-type cyclins phosphorylates pRB com- clin-dependent kinases (CDKs) and possibly target mitting the cell to enter S) is perpetually active them to respective substrate proteins within the throughout the cell cycle in these tumor lines. Ex- cell. CDK-mediated phosphorylation of specsc sets pression of cyclin D also may serve to discriminate of proteins drives the cell through particular phases Go vs. GI cells and, as an activation marker, to iden- or checkpoints of the cell cycle. During unper- tify the mitogenically stimulated cells entering the turbed growth of normal cells, the timing of expres- cell cycle. Differences in cyclin expression make it sion of several cyclins is discontinuous, occurring possible to discrirmna* te between cells having the at discrete and well-defined periods of the cell cy- same DNA content but residing at different phases cle. Immunocytochemical detection of cyclins in such as in G2vs. M or G,/M of a lower DNA ploidy vs. relation to cell cycle position (DNA content) by GI cells of a higher ploidy. The expression of cyclins multiparameter flow cytometry has provided a new D, E, A and B1 provides new cell cycle landmarks approach to cell cycle studies. -

Datasheet for CDK1-Cyclin B
of T161 is required for activation of the CDK1- Supplied in: 100 mM NaCl, 50 mM HEPES Specific Activity: ~1,000,000 units/mg cyclin B complex and is mediated by the CDK (pH 7.5 @ 25°C), 0.1 mM EDTA, 1 mM DTT, 0.01% CDK1-cyclin B activating kinase (CAK). During G2 phase, Brij 35 and 50% glycerol. Molecular Weight: CDK1 (34 kDa), cyclin B CDK1-cyclin B complex is held in an inactive state (48 kDa). The apparent molecular weight of cyclin by phosphorylation of CDK1 at the two negative Reagents Supplied with Enzyme: B on SDS-PAGE is about 60 kDa. 1-800-632-7799 regulatory sites, T14 and Y15 by CDK1 inhibitory 10X NEBuffer for Protein Kinases (PK). [email protected] protein kinases, Myt1 and Wee1 respectively. Quality Control Assays www.neb.com Dephosphorylation of T14 and Y15 by cell division Reaction Conditions: 1X NEBuffer for PK (NEB Protease Activity: After incubation of 100 units #B6022), supplemented with 200 µM ATP (NEB P6020S 032130114011 cycle 25 (CDC25) protein phosphatase in late G2 of CDK1-cyclin B with a standard mixture phase activates the CDK1-cyclin B complex and #P0756) and gamma-labeled ATP to a final specific of proteins for 2 hours at 30°C, no proteolytic activity of 100–500 µCi/µmol. Incubate at 30°C. triggers the initiation of mitosis. During expression activity could be detected by SDS-PAGE analysis. P6020S in insect cells, the recombiniant CDK1-cyclin B is 1X NEBuffer for PK: Phosphatase Activity: After incubation of 200 units 20,000 U/ml Lot: 0321301 activated in vivo by endogenous kinase (1–4). -

The Interplay Between Cyclin-B-Cdc2 Kinase (MPF)
COMMENTARY 257 The interplay between cyclin-B–Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes Ariane Abrieu1, Marcel Dorée2 and Daniel Fisher3,* 1Ludwig Institute for Cancer Research, UCSD, 9500 Gilman Drive, La Jolla, California 92093-0660, USA 2CRBM,UPR 1086 CNRS,1919 route de Mende, 34293 Montpellier Cedex 5, France 3IGH, UPR 1142 CNRS, 141 Rue de la Cardonille, 34396 Montpellier Cedex 5, France *Author for correspondance (e-mail: fi[email protected]) Journal of Cell Science 114, 257-267 © The Company of Biologists Ltd Summary Throughout oocyte maturation, and subsequently during events of meiotic maturation throughout the animal the first mitotic cell cycle, the MAP kinase cascade and kingdom, including the suppression of DNA replication, the cyclin-B–Cdc2 kinase are associated with the control of segregation of meiotic chromosomes, and the prevention of cell cycle progression. Many roles have been directly or parthenogenetic activation. Central to many of these events indirectly attributed to MAP kinase and its influence on appears to be the control by MAP kinase of cyclin cyclin-B–Cdc2 kinase in different model systems; yet a translation and degradation. principle theme does not emerge from the published literature, some of which is apparently contradictory. Interplay between these two kinases affects the major Key words: Cyclin B, Cdc2, MAP kinase, MPF, Oocyte meiosis Introduction including suppression of DNA replication between meiosis I In the animal kingdom, oocytes arrest the cell cycle at G2 phase and meiosis II, meiotic chromosome segregation and after replicated chromosomes have undergone meiotic pairing prevention of parthenogenetic activation. Here, we analyse the and recombination. -

Novel INK4 Proteins, P19 and P18, Are Specific Inhibitors of the Cyclin
MOLECULAR AND CELLULAR BIOLOGY, May 1995, p. 2672–2681 Vol. 15, No. 5 0270-7306/95/$04.0010 Copyright q 1995, American Society for Microbiology Novel INK4 Proteins, p19 and p18, Are Specific Inhibitors of the Cyclin D-Dependent Kinases CDK4 and CDK6 HIROSHI HIRAI,1 MARTINE F. ROUSSEL,1 JUN-YA KATO,1 RICHARD A. ASHMUN,1,2 1,3 AND CHARLES J. SHERR * Departments of Tumor Cell Biology1 and Experimental Oncology2 and Howard Hughes Medical Institute,3 St. Jude Children’s Research Hospital, Memphis, Tennessee 38105 Received 13 January 1995/Returned for modification 14 February 1995/Accepted 22 February 1995 Cyclin D-dependent kinases act as mitogen-responsive, rate-limiting controllers of G1 phase progression in mammalian cells. Two novel members of the mouse INK4 gene family, p19 and p18, that specifically inhibit the kinase activities of CDK4 and CDK6, but do not affect those of cyclin E-CDK2, cyclin A-CDK2, or cyclin B-CDC2, were isolated. Like the previously described human INK4 polypeptides, p16INK4a/MTS1 and p15INK4b/MTS2, mouse p19 and p18 are primarily composed of tandemly repeated ankyrin motifs, each ca. 32 amino acids in length. p19 and p18 bind directly to CDK4 and CDK6, whether untethered or in complexes with D cyclins, and can inhibit the activity of cyclin D-bound cyclin-dependent kinases (CDKs). Although neither protein interacts with D cyclins or displaces them from preassembled cyclin D-CDK complexes in vitro, both form complexes with CDKs at the expense of cyclins in vivo, suggesting that they may also interfere with cyclin-CDK assembly. -

Targeting Cyclin-Dependent Kinases in Human Cancers: from Small Molecules to Peptide Inhibitors
Cancers 2015, 7, 179-237; doi:10.3390/cancers7010179 OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Review Targeting Cyclin-Dependent Kinases in Human Cancers: From Small Molecules to Peptide Inhibitors Marion Peyressatre †, Camille Prével †, Morgan Pellerano and May C. Morris * Institut des Biomolécules Max Mousseron, IBMM-CNRS-UMR5247, 15 Av. Charles Flahault, 34093 Montpellier, France; E-Mails: [email protected] (M.P.); [email protected] (C.P.); [email protected] (M.P.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-04-1175-9624; Fax: +33-04-1175-9641. Academic Editor: Jonas Cicenas Received: 17 December 2014 / Accepted: 12 January 2015 / Published: 23 January 2015 Abstract: Cyclin-dependent kinases (CDK/Cyclins) form a family of heterodimeric kinases that play central roles in regulation of cell cycle progression, transcription and other major biological processes including neuronal differentiation and metabolism. Constitutive or deregulated hyperactivity of these kinases due to amplification, overexpression or mutation of cyclins or CDK, contributes to proliferation of cancer cells, and aberrant activity of these kinases has been reported in a wide variety of human cancers. These kinases therefore constitute biomarkers of proliferation and attractive pharmacological targets for development of anticancer therapeutics. The structural features of several of these kinases have been elucidated and their molecular mechanisms of regulation characterized in depth, providing clues for development of drugs and inhibitors to disrupt their function. However, like most other kinases, they constitute a challenging class of therapeutic targets due to their highly conserved structural features and ATP-binding pocket. -

SRY-Box Containing Gene 17 Regulates the Wnt/ß-Catenin
The Journal of Neuroscience, September 28, 2011 • 31(39):13921–13935 • 13921 Development/Plasticity/Repair SRY-Box Containing Gene 17 Regulates the Wnt/-Catenin Signaling Pathway in Oligodendrocyte Progenitor Cells Li-Jin Chew,1 Weiping Shen,1 Xiaotian Ming,1 Vladimir V. Senatorov Jr,1 Hui-Ling Chen,1 Ying Cheng,1 Elim Hong,1 Susan Knoblach,2 and Vittorio Gallo1 1Center for Neuroscience Research, and 2Center for Genetic Medicine Research, Children’s Research Institute, Children’s National Medical Center, Washington, DC 20010 The SRY-box (Sox) transcription factors regulate oligodendrocyte differentiation, but their signaling targets are largely unknown. We have identified a major signal transduction pathway regulated by Sox containing gene 17 (Sox17) in the oligodendrocyte lineage. Mi- croarrayanalysisinoligodendrocyteprogenitorcells(OPCs)afterSox17attenuationrevealedupregulatedgenesassociatedwithcellcycle control and activation of the Wingless and integration site (Wnt)/-catenin pathway. Sox17 knockdown also increases the levels of cyclin D1, Axin2, and activated -catenin. In OPCs, the expression pattern of Sox17, cyclin D1, and secreted Frizzled-related protein-1 in the presence of platelet-derived growth factor (PDGF) was coordinately accelerated by addition of thyroid hormone, indicating differentiation-induced regulation of Sox17 targets. In developing white matter, decreased total -catenin, activated -catenin, and cyclin D1 levels coincided with the peak of Sox17 expression, and immunoprecipitates showed a developmentally regulated interaction among Sox17, T-cell transcription factor 4, and -catenin proteins. In OPCs, PDGF stimulated phosphorylation of glycogen synthase 3 and the Wnt coreceptor LRP6, and enhanced -catenin-dependent gene expression. Sox17 overexpression inhibited PDGF-induced TOPFLASH and cyclin D1 promoter activity, and decreased endogenous cyclin D1, activated -catenin, as well as total -catenin levels.