The Cryoelectron Microscopy Structure of the Human CDK-Activating Kinase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gene Expression Profile of THZ1-Treated Nasopharyngeal Carcinoma Cell Lines Indicates Its Involvement in the Inhibition of the Cell Cycle
460 Original Article Gene expression profile of THZ1-treated nasopharyngeal carcinoma cell lines indicates its involvement in the inhibition of the cell cycle Lijuan Gao1,2,3#, Shuang Xia4#, Kunyi Zhang1,2,3#, Chengguang Lin1,2,3, Xuyu He4, Ying Zhang4 1Sun Yat-sen University Cancer Center, Sun Yat-sen University, Guangzhou, China; 2State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University, Guangzhou, China; 3Department of Radiation Oncology, Cancer Center, Sun Yat-sen University, Guangzhou, China; 4Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China Contributions: (I) Conception and design: L Gao, S Xia, K Zhang; (II) Administrative support: X He, Y Zhang; (III) Provision of study materials or patients: L Gao, S Xia, K Zhang; (IV) Collection and assembly of data: C Lin; (V) Data analysis and interpretation: C Lin, X He, Y Zhang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #The authors contributed equally to this work. Correspondence to: Xuyu He; Ying Zhang. Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, 106 Zhongshan 2nd road, Guangzhou 510080, China. Email: [email protected]; [email protected]. Background: The aim of this study was to identify downstream target genes and pathways regulated by THZ1 in nasopharyngeal carcinoma (NPC). Methods: The gene expression profile of GSE95750 in two NPC cell lines, untreated group and treated with THZ1 group, was analyzed. -

Cytotoxic Effects and Changes in Gene Expression Profile
Toxicology in Vitro 34 (2016) 309–320 Contents lists available at ScienceDirect Toxicology in Vitro journal homepage: www.elsevier.com/locate/toxinvit Fusarium mycotoxin enniatin B: Cytotoxic effects and changes in gene expression profile Martina Jonsson a,⁎,MarikaJestoib, Minna Anthoni a, Annikki Welling a, Iida Loivamaa a, Ville Hallikainen c, Matti Kankainen d, Erik Lysøe e, Pertti Koivisto a, Kimmo Peltonen a,f a Chemistry and Toxicology Research Unit, Finnish Food Safety Authority (Evira), Mustialankatu 3, FI-00790 Helsinki, Finland b Product Safety Unit, Finnish Food Safety Authority (Evira), Mustialankatu 3, FI-00790 Helsinki, c The Finnish Forest Research Institute, Rovaniemi Unit, P.O. Box 16, FI-96301 Rovaniemi, Finland d Institute for Molecular Medicine Finland (FIMM), University of Helsinki, P.O. Box 20, FI-00014, Finland e Plant Health and Biotechnology, Norwegian Institute of Bioeconomy, Høyskoleveien 7, NO -1430 Ås, Norway f Finnish Safety and Chemicals Agency (Tukes), Opastinsilta 12 B, FI-00521 Helsinki, Finland article info abstract Article history: The mycotoxin enniatin B, a cyclic hexadepsipeptide produced by the plant pathogen Fusarium,isprevalentin Received 3 December 2015 grains and grain-based products in different geographical areas. Although enniatins have not been associated Received in revised form 5 April 2016 with toxic outbreaks, they have caused toxicity in vitro in several cell lines. In this study, the cytotoxic effects Accepted 28 April 2016 of enniatin B were assessed in relation to cellular energy metabolism, cell proliferation, and the induction of ap- Available online 6 May 2016 optosis in Balb 3T3 and HepG2 cells. The mechanism of toxicity was examined by means of whole genome ex- fi Keywords: pression pro ling of exposed rat primary hepatocytes. -

Targeting Cyclin-Dependent Kinase 9 and Myeloid Cell Leukaemia 1 in MYC-Driven B-Cell Lymphoma
Targeting cyclin-dependent kinase 9 and myeloid cell leukaemia 1 in MYC-driven B-cell lymphoma Gareth Peter Gregory ORCID ID: 0000-0002-4170-0682 Thesis for Doctor of Philosophy September 2016 Sir Peter MacCallum Department of Oncology The University of Melbourne Doctor of Philosophy Submitted in total fulfilment of the degree of Abstract Aggressive B-cell lymphomas include diffuse large B-cell lymphoma, Burkitt lymphoma and intermediate forms. Despite high response rates to conventional immuno-chemotherapeutic approaches, an unmet need for novel therapeutic by resistance to chemotherapy and radiotherapy. The proto-oncogene MYC is strategies is required in the setting of relapsed and refractory disease, typified frequently dysregulated in the aggressive B-cell lymphomas, however, it has proven an elusive direct therapeutic target. MYC-dysregulated disease maintains a ‘transcriptionally-addicted’ state, whereby perturbation of A significant body of evidence is accumulating to suggest that RNA polymerase II activity may indirectly antagonise MYC activity. Furthermore, very recent studies implicate anti-apoptotic myeloid cell leukaemia 1 (MCL-1) as a critical survival determinant of MYC-driven lymphoma. This thesis utilises pharmacologic and genetic techniques in MYC-driven models of aggressive B-cell lymphoma to demonstrate that cyclin-dependent kinase 9 (CDK9) and MCL-1 are oncogenic dependencies of this subset of disease. The cyclin-dependent kinase inhibitor, dinaciclib, and more selective CDK9 inhibitors downregulation of MCL1 are used -

Cell Cycle–Regulated Phosphorylation of P220 by Cyclin E/Cdk2 in Cajal Bodies Promotes Histone Gene Transcription
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press Cell cycle–regulated phosphorylation of p220NPAT by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription Tianlin Ma,1,8 Brian A. Van Tine,4,5,8 Yue Wei,2 Michelle D. Garrett,6 David Nelson,7 Peter D. Adams,7 Jin Wang,1,3 Jun Qin,1,3 Louise T. Chow,4 and J. Wade Harper1,2,9 1Department of Biochemistry and Molecular Biology, 2Department of Molecular Physiology and Biophysics, 3Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas 77030, USA; 4Department of Biochemistry and Molecular Genetics, 5Department of Pathology, University of Alabama, Birmingham, Alabama 35294, USA; 6CRC Centre for Cancer Therapeutics, Institute of Cancer Research, Sutton, SM2 5NG, United Kingdom; 7Fox Chase Cancer Center, Philadelphia, Pennsylvania 19111, USA Cyclin E/Cdk2 acts at the G1/S-phase transition to promote the E2F transcriptional program and the initiation of DNA synthesis. To explore further how cyclin E/Cdk2 controls S-phase events, we examined the subcellular localization of the cyclin E/Cdk2 interacting protein p220NPAT and its regulation by phosphorylation. p220 is localized to discrete nuclear foci. Diploid fibroblasts in Go and G1 contain two p220 foci, whereas S- and G2-phase cells contain primarily four p220 foci. Cells in metaphase and telophase have no detectable focus. p220 foci contain cyclin E and are coincident with Cajal bodies (CBs), subnuclear organelles that associate with histone gene clusters on chromosomes 1 and 6. Interestingly, p220 foci associate with chromosome 6 throughout the cell cycle and with chromosome 1 during S phase. -

Pharmacodynamic Effects of Seliciclib, an Orally Administered
Cancer Therapy: Clinical Pharmacodynamic Effects of Seliciclib, an Orally Administered Cell Cycle Modulator, in Undifferentiated Nasopharyngeal Cancer Wen-Son Hsieh,4 Ross Soo,1Bee-Keow Peh,2 Thomas Loh,4 Difeng Dong,3 Donny Soh,5 Lim-SoonWong,3,5 Simon Green,6 Judy Chiao,6 Chun-Ying Cui,1Yo k e -F o n g L a i , 4 Soo-Chin Lee,1Benjamin Mow,1 Richie Soong,2 Manuel Salto-Tellez,2 and Boon-Cher Goh1, 2 Abstract Purpose: Cell cycle dysregulation resulting in expression of antiapoptotic genes and uncontrolled proliferation is a feature of undifferentiated nasopharyngeal carcinoma. The pharmacodynamic effects of seliciclib, a cyclin-dependent kinase (CDK) inhibitor, were studied in patients with nasopharyngeal carcinoma. Experimental Design: Patients with treatment-naI«ve locally advanced nasopharyngeal carci- noma received seliciclib at 800 mg or 400 mg twice daily on days1 to 3 and 8 to12.Paired tumor samples obtained at baseline and on day 13 were assessed by light microscopy, immunohisto- chemistry, and transcriptionalprofiling using real-time PCR low-density array consisting of apanel of 380 genes related to cell cycle inhibition, apoptosis, signal transduction, and cell proliferation. Results: At 800 mg bd, one patient experienced grade 3 liver toxicity and another had grade 2 vomiting; no significant toxicities were experienced in 13 patients treated at 400 mg bd. Seven of fourteen evaluable patients had clinical evidence of tumor reduction. Some of these responses were associated with increased tumor apoptosis, necrosis, and decreases in plasma EBV DNA posttreatment. Reduced protein expression of Mcl-1, cyclin D1, phosphorylated retinoblastoma protein pRB (T821), and significant transcriptional down-regulation of genes related to cellular proliferation and survival were shown in some patients posttreatment, indicative of cell cycle modulation by seliciclib, more specifically inhibition of cdk2/cyclin E, cdk7/cyclin H, and cdk9/cyclinT. -

Datasheet for CDK1-Cyclin B
of T161 is required for activation of the CDK1- Supplied in: 100 mM NaCl, 50 mM HEPES Specific Activity: ~1,000,000 units/mg cyclin B complex and is mediated by the CDK (pH 7.5 @ 25°C), 0.1 mM EDTA, 1 mM DTT, 0.01% CDK1-cyclin B activating kinase (CAK). During G2 phase, Brij 35 and 50% glycerol. Molecular Weight: CDK1 (34 kDa), cyclin B CDK1-cyclin B complex is held in an inactive state (48 kDa). The apparent molecular weight of cyclin by phosphorylation of CDK1 at the two negative Reagents Supplied with Enzyme: B on SDS-PAGE is about 60 kDa. 1-800-632-7799 regulatory sites, T14 and Y15 by CDK1 inhibitory 10X NEBuffer for Protein Kinases (PK). [email protected] protein kinases, Myt1 and Wee1 respectively. Quality Control Assays www.neb.com Dephosphorylation of T14 and Y15 by cell division Reaction Conditions: 1X NEBuffer for PK (NEB Protease Activity: After incubation of 100 units #B6022), supplemented with 200 µM ATP (NEB P6020S 032120413041 cycle 25 (CDC25) protein phosphatase in late G2 of CDK1-cyclin B with a standard mixture phase activates the CDK1-cyclin B complex and #P0756) and gamma-labeled ATP to a final specific of proteins for 2 hours at 30°C, no proteolytic activity of 100–500 µCi/µmol. Incubate at 30°C. triggers the initiation of mitosis. During expression activity could be detected by SDS-PAGE analysis. P6020S in insect cells, the recombiniant CDK1-cyclin B is 1X NEBuffer for PK: Phosphatase Activity: After incubation of 200 units 20,000 U/ml Lot: 0321204 activated in vivo by endogenous kinase (1–4). -
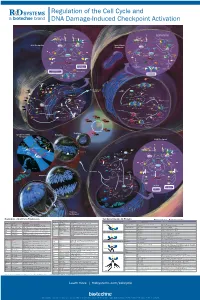
Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation
RnDSy-lu-2945 Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation IR UV IR Stalled Replication Forks/ BRCA1 Rad50 Long Stretches of ss-DNA Rad50 Mre11 BRCA1 Nbs1 Rad9-Rad1-Hus1 Mre11 RPA MDC1 γ-H2AX DNA Pol α/Primase RFC2-5 MDC1 Nbs1 53BP1 MCM2-7 53BP1 γ-H2AX Rad17 Claspin MCM10 Rad9-Rad1-Hus1 TopBP1 CDC45 G1/S Checkpoint Intra-S-Phase RFC2-5 ATM ATR TopBP1 Rad17 ATRIP ATM Checkpoint Claspin Chk2 Chk1 Chk2 Chk1 ATR Rad50 ATRIP Mre11 FANCD2 Ubiquitin MDM2 MDM2 Nbs1 CDC25A Rad50 Mre11 BRCA1 Ub-mediated Phosphatase p53 CDC25A Ubiquitin p53 FANCD2 Phosphatase Degradation Nbs1 p53 p53 CDK2 p21 p21 BRCA1 Ub-mediated SMC1 Degradation Cyclin E/A SMC1 CDK2 Slow S Phase CDC45 Progression p21 DNA Pol α/Primase Slow S Phase p21 Cyclin E Progression Maintenance of Inhibition of New CDC6 CDT1 CDC45 G1/S Arrest Origin Firing ORC MCM2-7 MCM2-7 Recovery of Stalled Replication Forks Inhibition of MCM10 MCM10 Replication Origin Firing DNA Pol α/Primase ORI CDC6 CDT1 MCM2-7 ORC S Phase Delay MCM2-7 MCM10 MCM10 ORI Geminin EGF EGF R GAB-1 CDC6 CDT1 ORC MCM2-7 PI 3-Kinase p70 S6K MCM2-7 S6 Protein Translation Pre-RC (G1) GAB-2 MCM10 GSK-3 TSC1/2 MCM10 ORI PIP2 TOR Promotes Replication CAK EGF Origin Firing Origin PIP3 Activation CDK2 EGF R Akt CDC25A PDK-1 Phosphatase Cyclin E/A SHIP CIP/KIP (p21, p27, p57) (Active) PLCγ PP2A (Active) PTEN CDC45 PIP2 CAK Unwinding RPA CDC7 CDK2 IP3 DAG (Active) Positive DBF4 α Feedback CDC25A DNA Pol /Primase Cyclin E Loop Phosphatase PKC ORC RAS CDK4/6 CDK2 (Active) Cyclin E MCM10 CDC45 RPA IP Receptor -

Human P14arf-Mediated Cell Cycle Arrest Strictly Depends on Intact P53 Signaling Pathways
Oncogene (2002) 21, 3207 ± 3212 ã 2002 Nature Publishing Group All rights reserved 0950 ± 9232/02 $25.00 www.nature.com/onc Human p14ARF-mediated cell cycle arrest strictly depends on intact p53 signaling pathways H Oliver Weber1,2, Temesgen Samuel1,3, Pia Rauch1 and Jens Oliver Funk*,1 1Laboratory of Molecular Tumor Biology, Department of Dermatology, University of Erlangen-Nuremberg, 91052 Erlangen, Germany; 2Regulation of Cell Growth Laboratory, National Cancer Institute, Frederick, Maryland, MD 21702-1201, USA The tumor suppressor ARF is transcribed from the INK4a/ 4 and 6 activities, thus leading to decreased phosphor- ARF locus in partly overlapping reading frames with the ylation of RB and to G1 arrest. Cells that are de®cient CDK inhibitor p16Ink4a. ARF is able to antagonize the for RB are resistant to p16Ink4a-mediated cell cycle MDM2-mediated ubiquitination and degradation of p53, arrest (Sherr and Roberts, 1995; Weinberg, 1995). leading to either cell cycle arrest or apoptosis, depending on ARF is also a cell cyle inhibitor. It does not directly the cellular context. However, recent data point to inhibit CDKs but interferes with the function of additional p53-independent functions of mouse p19ARF. MDM2 to destabilize p53. ARF may be activated by Little is known about the dependency of human p14ARF aberrant activation of oncoproteins such as Ras function on p53 and its downstream genes. Therefore, we (Palmero et al., 1998), c-myc (Zindy et al., 1998), analysed the mechanism of p14ARF-induced cell cycle arrest E1A (de Stanchina et al., 1998), Abl (Radfar et al., in several human cell types. -

21-Containing C.Yclin Kinases Exist in Oth Acnve and Lnacnve States
Downloaded from genesdev.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press 21-containing c.yclin kinases exist in oth acnve and lnacnve states Hui Zhang, Gregory J. Hannon, and David Beach Howard Hughes Medical Institute, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724 USA In normal fibroblasts CDKs exist predominantly in p21/PCNA/cyclin/CDK quaternary complexes, whereas in p53-deficient cells, p21 expression is depressed and the kinases are reduced to a cyclin/CDK binary state, p21 is a universal cyclin kinase inhibitor, but we show here that p21-containing complexes exist in both catalytically active and inactive forms. This finding challenges the current view that active cyclin kinases function only in the binary state and reveals the subtlety with which tumor-suppressor proteins modulate the cell cycle. [Key Words: p21-containing cyclin kinase; cell cycle progression; human fibroblasts; quaternary complex; kinase inhibitor] Received May 12, 1994; revised version accepted June 16, 1994. Much of our current understanding of the regulation of their relative affinities vary with each enzyme (Gu et al. the cell division cycle has emerged from studies of a 1993; Harper et al. 1993; Xiong et al. 1993b). Further- family of protein kinases [cdc2, CDC28, and generically more, several lines of evidence suggest that p21 expres- cyclin-dependent kinase (CDK)] and their inhibitors and sion is regulated by the p53 tumor-suppressor protein activators (for review, see Sherr 1993). A critical step in (E1-Deiry et al. 1993; Xiong et al. 1993b). Thus, cells understanding cell cycle control was the discovery that derived from certain p53-deficient Li-Fraumeni patients CDKs interact with cyclins, proteins that serve as essen- lack p21 associated with cyclin kinases (Xiong et al. -

NF-Y Binds to Both G1- and G2-Specific Cyclin Promoters; a Possible Role in Linking CDK2/Cyclin a to CDK1/Cyclin B
BMB reports NF-Y binds to both G1- and G2-specific cyclin promoters; a possible role in linking CDK2/Cyclin A to CDK1/Cyclin B Hee-Don Chae#, Jungbin Kim# & Deug Y. Shin* Department of Microbiology, Dankook University College of Medicine, Cheonan, 330-714, Korea We previously reported that CDK2/Cyclin A can phosphor- standing of cell growth and division. ylate and activate the transcription factor NF-Y. In this study, It is well established that retinoblastoma (Rb) tumor sup- we investigated a potential regulatory role for NF-Y in the tran- pressor proteins coordinate the sequential activation of CDK4 scription of Cyclin A and other cell cycle regulatory genes. and CDK2 during the G1/S transition (3, 4). The CDK4/Cyclin Gel-shift assays demonstrate that NF-Y binds to CCAAT se- D complex phosphorylates Rb, promoting Cyclin E expression quences in the Cyclin A promoter, as well as to those in the through its E2F-dependent transcriptional activation and dis- promoters of cell cycle G2 regulators such as CDC2, Cyclin B ruption of the Rb-HDAC complex, culminating in CDK2 kin- and CDC25C. Furthermore, expression of Cyclin A increases ase activation as S phase progresses (5, 6). In contrast, the fac- NF-Y’s affinity for CCAAT sequences in the CDC2 promoter; tors involved in the handoff from CDK2 to CDK1 remain however, Cyclin A’s induction of CDC2 transcription is antag- unidentified. However, previous work by our group and others onized by p21, an inhibitor of CDK2/Cyclin A. These results demonstrates that overexpression of p53 blocks the G2/M tran- suggest a model wherein NF-Y binds to and activates tran- sition (7, 8) and inhibits cell cycle-dependent transcription of scription from the Cyclin A promoter, increasing cellular levels CDC2 and CCNB genes with consequent CDK1 inactivation of Cyclin A/CDK2 and potentiating NF-Y’s capacity for tran- (7). -

Cytometry of Cyclin Proteins
Reprinted with permission of Cytometry Part A, John Wiley and Sons, Inc. Cytometry of Cyclin Proteins Zbigniew Darzynkiewicz, Jianping Gong, Gloria Juan, Barbara Ardelt, and Frank Traganos The Cancer Research Institute, New York Medical College, Valhalla, New York Received for publication January 22, 1996; accepted March 11, 1996 Cyclins are key components of the cell cycle pro- gests that the partner kinase CDK4 (which upon ac- gression machinery. They activate their partner cy- tivation by D-type cyclins phosphorylates pRB com- clin-dependent kinases (CDKs) and possibly target mitting the cell to enter S) is perpetually active them to respective substrate proteins within the throughout the cell cycle in these tumor lines. Ex- cell. CDK-mediated phosphorylation of specsc sets pression of cyclin D also may serve to discriminate of proteins drives the cell through particular phases Go vs. GI cells and, as an activation marker, to iden- or checkpoints of the cell cycle. During unper- tify the mitogenically stimulated cells entering the turbed growth of normal cells, the timing of expres- cell cycle. Differences in cyclin expression make it sion of several cyclins is discontinuous, occurring possible to discrirmna* te between cells having the at discrete and well-defined periods of the cell cy- same DNA content but residing at different phases cle. Immunocytochemical detection of cyclins in such as in G2vs. M or G,/M of a lower DNA ploidy vs. relation to cell cycle position (DNA content) by GI cells of a higher ploidy. The expression of cyclins multiparameter flow cytometry has provided a new D, E, A and B1 provides new cell cycle landmarks approach to cell cycle studies. -

Expression and Subcellular Localization of Cyclin H in Bombyx Mori
Life Science Journal 2013;10(3) http://www.lifesciencesite.com Expression and subcellular localization of cyclin H in Bombyx mori Zhang Haihua, Jiang Yan, Chen Chen, Zhang Hanming,Yu Wei, Zhang Yaozhou, Tong Fudan* Biochemistry Institute of Zhejiang Sci-tech University, Hangzhou, Zhejiang 310018, China [email protected] Abstract:Cyclin H is normally associated with cyclin-dependent kinase 7 (Cdk7). However, cyclin H is also a substrate of protein kinase 2, a ubiquitously expressed serine/threonine protein kinase required for cell viability and cell-cycle progression. Studies of cyclin H have focused mainly on vertebrates, and little is known about its expression in the silkworm Bombyx mori. In the present study, the cDNA sequence of cyclin H, which encodes a protein of 250 amino acid residues, was identified in a silkworm pupa cDNA library constructed in our laboratory (GenBank No. AV406047). The open reading frame was 753-bp long and encodes a protein (BmCyc H) with a conserved domain Ccl 1 motif. Recombinant BmCyc H was expressed in Escherichia coli BL21(DE3), purified, and used to prepare polyclonal antibodies. Western blotting revealed that the protein was expressed in various tissues in fifth instar larvae, and also in each differentiated growth stage of B. mori, with the highest expression in the pupa and spiracle of the fifth instar larvae. The lowest levels were detected in eggs, ovaries, and heart in fifth instar larvae. The results of real-time quantitative polymerase chain reaction revealed that BmCyc H mRNA was widespread in different tissues and growth stages of B. mori, with the highest expression levels in the moth and pupa and in the spiracles, fatty body, and heart of the fifth instar larvae, with the lowest levels in eggs, and in Malpighian tubules, ovaries, epidermis, gut, and silk gland in fifth instar larvae.