Synthesis and Process Optimization of Electrospun PEEK-Sulfonated Nanofibers by Response Surface Methodology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 1. SOME NAMES and ABBREVIATIONS of Plastics and Elastomers
TABlE 1. SOME NAMES AND ABBREVIATIONS OF PlASTICS AND ElASTOMERS. Common name Abbreviation Acetal (homopolymer and copolymer) POM-H and POM-K Acrylate styrene acrylonitrile ASAorAAS Acrylate modified styrene acrylonitrile ASAorAAS Acrylic acid ester rubber ACM Acrylonitrile butadiene rubber or nitrile butadiene rubber NBR Acrylonitrile butadiene styrene ABS Acrylonitrile styrene/chlorinated polyethylene ACS Acrylonitrile methyl methacrylate AMMA Acrylonitrile styrene/EPR rubber or, acrylonitrile ethylene propylene styrene AES Alpha methyl styrene AMS Atactic polypropylene APPorPP-A Butadiene rubber or, cis-1,4-polybutadiene rubber or, polybutadiene rubber BR Butadiene styrene block copolymer BDS Butyl rubber IIR Bulk molding compound BMC Casein formaldehyde CF Cellulose acetate CA Cellulose acetate butyrate CAB Cellulose acetate propionate CAP Cellulose nitrate CN Chlorinated polyethylene CPEorCM Chlorinated polyvinyl chloride CPVC or, PVC-C Chloro-polyethylene or, chlorinated polyethylene. CM or CPE or, PE-C Chloroprene rubber or, polychloroprene rubber CR Chlorotrifluoroethylene ethylene copolymers ECfFE Cis-polyisoprene or, cis-1,4-polyisoprene IR Coumarone indene resins CIR Diallyl phthalate DAP Diallyl isophthalate DAIP Dough molding compound DMC Elastomeric alloy melt processable rubber EA-MPR Elastomeric alloy thermoplastic vulcanizate EA-TPV Epichlohydrin rubber CHR Epoxy or, epoxide EP Epoxy or, epoxide, with glass fiber EPGF Ethyl cellulose EC Ethylene acryic acid EAA Ethylene propylene diene monomer (an EPR terpolymer) EPDM -

Synthetic Polymeric Materials for Bone Replacement
Review Synthetic Polymeric Materials for Bone Replacement Mônica Rufino Senra and Maria de Fátima Vieira Marques * Technology Center, Bloco J, Instituto de Macromoleculas Professora Eloisa Mano, Universidade Federal do Rio de Janeiro, IMA-UFRJ, Av. Horacio Macedo, 2030, Rio de Janeiro RJ 21941-598, Brazil; [email protected] * Correspondence: [email protected] Received: 17 November 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: Some treatment options available to repair bone defects are the use of autogenous and allogeneic bone grafts. The drawback of the first one is the donor site’s limitation and the need for a second operation on the same patient. In the allograft method, the problems are associated with transmitted diseases and high susceptibility to rejection. As an alternative to biological grafts, polymers can be used in bone repair. Some polymers used in the orthopedic field are poly(methyl methacrylate), poly(ether-ether-ketone), and ultra-high molecular weight polyethylene (UHMWPE). UHMWPE has drawn much attention since it combines low friction coefficient and high wear and impact resistance. However, UHMWPE is a bioinert material, which means that it does not interact with the bone tissue. UHMWPE composites and nanocomposites with hydroxyapatite (HA) are widely studied in the literature to mitigate these issues. HA is the main component of the inorganic phase in the natural bone, and the addition of this bioactive filler to the polymeric matrix aims to mimic bone composition. This brief review discusses some polymers used in orthopedic applications, focusing on the UHMWPE/HA composites as a potential bone substitute. Keywords: poly(methyl methacrylate); poly(ether-ether-ketone); ultra-high molecular weight polyethylene; hydroxyapatite; orthopedic applications 1. -

Mechanical Properties Thermoplastic Laminates of Polycarbonate
Scienc ile e & Singh et al., J Textile Sci Eng 2018, 8:4 xt e E T n : g DOI 10.4172/2165-8064.1000366 f i o n l e a e n r r i n u g o Journal of Textile Science & Engineering J ISSN: 2165-8064 Research Article Article OpenOpen Access Access Mechanical Properties Thermoplastic Laminates of Polycarbonate- Polyetherimide Blend with Glass Fibers Singh JC1*, Srivastava M2, Singh RK2 and Yadaw SB3 1Indian Institute of Technology, New Delhi, India 2Central Institute of Plastics Engineering and Technology, Hajipur, India 3Defence Material & Stores Research & Development Establishment, Kanpur, India Abstract Thermoplastic Laminates of Polycarbonate (PC)/Polyetherimide (PEI) blend with glass fibers were prepared by solution blending of PC & PEI in different ratio of 100/0, 95/5, 90/10, 85/15 and 80/20 using Dichloromethane as solvent followed by applying the resulting blend solutions over the cut sheet size (30 × 25 cm) of woven mat glass fibers & dried overnight, then stacked up 12 flies of the impregnates and consolidated in a hot press at 300ºC and 3-4 ton (30 kg/cm2) pressure for a duration of one hour & cooled. The laminates of PC/PEI blends/woven mat glass fibers were studied for their mechanical properties viz: Tensile, Flexural, Impact and Inter Laminar Shear Strength and thermo-mechanical properties viz: TGA, DSC, DMA as well as examination of morphology by SEM. The mechanical properties of laminates of PC/PEI blends/woven mat glass fibers shows an increasing trend in Inter Laminar Shear Strength, Tensile and Flexural Strength upto 20% PEI while Impact Strength shows an increase upto 10% and decreases while the percentage of PEI increases. -
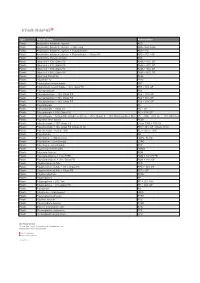
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Impact Strength of 3D Printed Polyether-Ether-Ketone (PEEK)
Solid Freeform Fabrication 2019: Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium – An Additive Manufacturing Conference Impact Strength of 3D Printed Polyether-ether-ketone (PEEK) Haijun Gong1,*, Xiaodong Xing2, Jan Nel1 1Department of Manufacturing Engineering, Georgia Southern University, Statesboro GA, USA 2College of Mechanical and Electrical Engineering, Harbin Engineering University, Harbin, China *Corresponding author ([email protected]) Abstract Polyether-ether-ketone (PEEK) is a high-performance thermoplastic with high heat-, high chemical-, high water-, and high wear-resistance. Its strength and durability also make it highly accepted for a range of industrial applications. 3D printing of PEEK filaments offers a unique quality and flexibility in making PEEK parts for low-volume production or special designs. This study investigates the impact strength of 3D printed PEEK materials. The specimens are fabricated using a fused deposition modeling (FDM) based 3D printer and tested by a pendulum impact tester in compliance with ASTM standard. The testing result is discussed with respect to the processing parameters and the annealing treatment. Impact strength comparison of PEEK materials manufactured by 3D printing and by conventional production is also conducted. Instruction Polyether-ether-ketone (PEEK) is a semi-crystalline polymer, which was developed in 1978. The PEEK is widely used as a high-performance thermoplastic due to its high heat-, high chemical-, high water-, high wear-resistance. Especially, PEEK and its varieties have been adopted for different biomedical treatment such as dental implants, orthopedics and maxillofacial surgery [1,2]. Its strength and durability also make it highly accepted for a range of industrial applications. For example, many automotive applications require to work at a temperature more than 120 °C. -

Polymer Properties and Classification
Polymer Characterization LDPE Polyethylene low density HDPE Polyethylene high density ABS Acrylonitrile-butadiene-styrene SAN Styrene-acrylonitrile copolymer EVA Polyethylene co-vinyl acetate PVA Polyvinyl acetate PerkinElmer Solutions for Polymer Characterization Tg(ºC): -130 to 100 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): -130 to 100 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): 110 to 125 CpJ/(g*K): 1,25 to 1,7 Tg(ºC): 95 to 110 CpJ/(g*K): 1,2 Tg(ºC): -45 to 20 CpJ/(g*K): 2,3 Tg(ºC): 25 to 35 CpJ/(g*K): - Tm(ºC): 100 to 120 DHf (J/g): - Tm(ºC): 130 to 140 DHf (J/g): 293 Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): 30 to 100 DHf (J/g): 10 to 100 Tm( ºC ): - DHf (J/g): - Td(ºC): 490 to 500 Td(ºC): 490 to 500 Td(ºC): 420 Td(ºC): 420 Td(ºC): 480 Td(ºC): - PP Polypropylene PS PMMA Polymethylmethacrylate PBMA CA Polystyrene Polybuthylmethacrylate Cellulose acetate EP Epoxy resin Molecular Spectroscopy FTIR Differential Scanning Calorimetry Tg(ºC): -20 to -5 CpJ/(g*K): 1,8 Tg(ºC): 90 to 110 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): 85 to 100 CpJ/(g*K): 1,45 to 1,5 Tg(ºC): 15 to 25 CpJ/(g*K): - Tg(ºC): 45 to 60 CpJ/(g*K): - Tg(ºC): 50 to 200 CpJ/(g*K): 1,6 to 2,1 Identify and quantitate organic molecules and compounds, Glass transition & melting temperatures, crystallinity, heat of Understand chemical & physical composition of laminates & fusion, reaction rates, specific heat & heat capacity, curing, Tm(ºC): 165 to 175 DHf (J/g): 207 Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm( ºC): - DHf (J/g): - adhesives , Troubleshoot -

Dielectric Spectroscopy and Thermally Stimulated Discharge Current in PEEK Film
Volume 28 (2003) número 2 www.scielo.br/eq Dielectric spectroscopy and thermally stimulated discharge current in PEEK film W. K. Sakamoto Departamento de Física e Química – FEG/UNESP - CEP 15385-000 Ilha Solteira –SP – Brasil Abstract: The complex permittivity of films of polyether ether ketone (PEEK) has been investigated over a wide range of frequency. There is no relaxation peak in the range of 1Hz to 105 Hz but in the low- frequency side (10-4 Hz) there is an evidence of a peak that also can be observed by thermally stimulated discharge current measurements. That peak is related with the glass transition temperature (Tg) of the polymer. The activation energy of the relaxation was found to be 0.44 eV, similar to that of several synthetic polymers. Space charges are important in the conduction mechanism as shown by discharging transient. Keywords: dielectric loss; thermally stimulated discharge current; polymer; activation energy; glass transition temperature. Introduction Although PEEK has already been used as insulating material and its electrical and dielectric Polyether ether ketone (PEEK) is an properties have been reported [4, 5], this paper aromatic polyether. It has been developed by I. C. reports results of dielectric spectroscopy in the wide I. Petrochemicals and Plastic Division as a high- frequency range of 10-5 Hz to 105 Hz and the nature temperature engineering thermoplastic [1]. of the thermally stimulated discharge current Because of the symmetry of the benzene rings (TSDC) in the temperature range from 10 oC to along the polymer backbone, PEEK offers above glass transition temperature (Tg) is analyzed. -

A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone
polymers Article A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone Franco Dominici 1,* , Debora Puglia 1 , Francesca Luzi 1, Fabrizio Sarasini 2 , Marco Rallini 1 and Luigi Torre 1 1 Civil and Environmental Engineering Department, University of Perugia, Strada di Pentima 4, 05100 Terni, Italy; [email protected] (D.P.); [email protected] (F.L.); [email protected] (M.R.); [email protected] (L.T.) 2 Department of Chemical Engineering Materials Environment, Sapienza-Università di Roma and UdR INSTM, Via Eudossiana 18, 00184 Roma, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0744-492910 Received: 27 November 2019; Accepted: 12 December 2019; Published: 14 December 2019 Abstract: Poly(ether ether ketone) (PEEK)-based nanocomposites have been realized with incorporation (0–30 wt %) of anhydrous calcium terephthalate salts (CATAS), synthetized by reaction of terephtalic acid with the metal (Ca) oxide, by means of a melt processing. Their structure, morphology, thermal, and mechanical properties have been investigated. Scanning electron microscopy observations confirmed homogeneous dispersion of nanometer-sized fillers and a toughened fracture morphology even at the higher content, while thermal characterization confirmed an unvaried thermal stability and unmodified crystalline structure of the reference PEEK matrix. A negligible nucleating effect was evidenced, while a blocking effect of the amorphous phase fraction provide composites with increased stiffness, confirmed by enhanced values of G’ and shifts of glass transition peak to higher temperatures, for restriction in chain mobility imposed by CATAS. The proposed solutions aimed to enlarge the application range of high performance costly PEEK-based composites, by using thermally stable nanofillers with limited costs and easily controllable synthesis phase. -

A Cid–Base and Surface Energy Characterization of Grafted
Journal of Chromatography A, 969 (2002) 73–80 www.elsevier.com/locate/chroma A cid–base and surface energy characterization of grafted polyethylene using inverse gas chromatography Petra Uhlmann* , Steffen Schneider1 Department of Biocompatible Materials, Institute of Polymer Research, Hohe Strasse 6, D-01069 Dresden, Germany Abstract For a specific design of interfaces, i.e. in composites and blends, it is essential to know the surface thermodynamics of the components. Polyethylene grafted with maleic anhydride and maleic anhydride–styrene mixtures, respectively, was the component of interest of our investigations. Inverse gas chromatography (IGC) at infinite dilution was shown to be an appropriate method to evaluate the dispersive and acid–base surface characteristics although there is an influence of bulk absorption and morphology when performing IGC above the glass transition temperature of the polymer. 2002 Elsevier Science B.V. All rights reserved. Keywords: Surface characteristics; Inverse gas chromatography; Thermodynamic parameters; Adsorption; Polyethylene 1 . Introduction economically and environmentally advantageous, the consistency of the polymer is not changed [1]. Polyalkenes have some excellent properties which The mechanical properties of such materials with are advantageous for their technical application, but two or more components are strongly influenced by normally they have non-polar surface characteristics, the properties of the components on the one hand a fact which is limiting their field of use. In order to and by the properties of the interface or interphase make blends and composites or to improve the on the other. In cases where there is no chemical adhesion of polyalkenes, it is necessary to introduce bonding between the components, and the surface of polar groups into the interface between the com- the material is smooth, the interaction between the ponents and the surface, respectively. -

Investigation of Tribological Behavior of PEEK and PEEK Composites at Elevated Temperature
International Journal of Current Engineering and Technology E-ISSN 2277 – 4106, P-ISSN 2347 - 5161 ® ©2014 INPRESSCO , All Rights Reserved Available at http://inpressco.com/category/ijcet Research Article Investigation of Tribological behavior of PEEK and PEEK Composites at Elevated Temperature S.B.BhoyarȦ*, R.L.Kadu Ȧ, P.M. KarandikarȦ and R.R.KhardeȦ ADepartment of Mechanical Engineering,Pravara Rural Engineering College ,Loni,413736, India Accepted 10 April 2014, Available online 16 April 2014, Vol.4, No.2 (February 2014) Abstract In the petroleum , chemical industry and coalmines where high temperature is often seen, special materials have to be used. The high wear rate at high temperature is a serious problem in large number of industrial applications such as elevated temperature compressor piston rings and bearings. Meanwhile to meet the combination of light weight and high strength demands polymer-based materials are increasingly applied in many industries .However, at temperatures above 180°C is a challenge for most of the polymer composites. PEEK composites are expected to have excellent high- temperature tribological properties due to their outstanding mechanical and thermal performances above 180°C, where polytetrafluoroethylene (PTFE) composites will meet severe creep behaviors. To improve the tribological properties of high temperature resistance thermoplastic polyether-ether-ketone (PEEK) and PTFE, reinforced with short carbon fibre (SFC), graphite flakes, and sub- micro particles of MoS2 were investigated in dry sliding conditions. Friction and wear experiments were conducted on pin-on-disc apparatus, using composite pins against polished EN8 steel counterparts. Test performed within the p v-ranges at room temperature and elevated temperature (up to 300 °C). -

New Focus on Peek
NEW FOCUS ON PEEK INTRODUCTION Polyether ether ketone, or PEEK, is a high-performance and highly popular engineered thermoplastic. PEEK is a member of the polyaryletherketone (PAEK) family which includes other familiar semi-crystalline polymers such as polyetherketone (PEK), polyetherketoneketone (PEKK), and polyetherether-ketoneketone (PEEKK). This family of polymers is known for such performance characteristics as excellent mechanical properties and chemical resistance. PAEK family polymers retain these attributes even at high temperatures, and thus their thermal stability has become one of their hallmarks. Although relatively new to the commercial synthetic polymer market (ca. 1978), PEEK has now found its way into such diverse applications as tubing, molded parts ranging from bearings to plates to gears, insulation such as motors parts and coating for wires, to prosthetics and implantables for use within the human body. For performance plastics, PEEK is often compared to fluoropolymers because it shares several high-performing and beneficial features with several of the best-performing fluoropolymers – including a service temperature up to 260 °C (500 °F). PEEK, however, provides more diversity options to attain properties that may be out of reach for some fluoropolymers. Today, PEEK is the most widely commercialized PAEK family polymer, and new uses continue to come to the fore. SYNTHESIS AND STRUCTURE PEEK was first synthesized in 1977 and was industrialized in 1981 [1, 2]. PEEK is produced most immediately from difluorobenzophenone and disodium hydroquinone (Fig. 1). Often viewed in a class with fluoropolymers such as PTFE or PFA, PEEK polymer, however, does not contain fluorine. Initial synthesis of PEEK was difficult due to its insolubility in most organic solvents [2, 3]. -

Polyglycolic Acid (PGA) Resin
The Pursuit of Excellence High Performance Polyester Polyglycolic acid (PGA) Resin KUREHA CORPORATION KUREHA ENERGY PGA Department SOLUTIONS LLC 3-3-2 Nihonbashi-Hamacho, Chuo-ku, 3151 Briarpark Drive, Suite 1050, Tokyo, Japan 103-8552 Houston, Texas 77042 , USA Tel: +81-3-3249-4650 Tel: +1-713-893-0730 http://www.kuredux.com/ Dec, 2019 Kuredux -Polyglycolic acid(PGA) - Polyglycolic acid (PGA) is a biodegradable polyester resin that possesses several remarkable characteristics. As the mass production was difficult, PGA resin had been used in the limited field as medical suture until 2010. Kureha Corporation’s proprietary polymerization technology resulted in the world’s first industrial scale PGA manufacturing facility, allowing the introduction and Molecular Structure of ® first commercial widespread use of Kuredux as a new polymer offering Polyglycolic acid to industry. Biodegradability, Hydrolyzability Characteristics of Kuredux® High Strength and Modulus High abrasion resistance High solvent resistance Biodegradability Kuredux® is a fully compostable plastic, satisfying the test protocols for ISO 14855 as well as ASTM D6400 and EN13432, and certified as a biodegradable plastic by TUV(EU) and JBPA/GreenPla (Japan). <Tests based on ISO14855 protocols> 100 80 ― ― HO-CH -C= -OH O-CH -C= 2 60 ( 2 ) n CO Cellulose 2 H O O O 2 Kuredux ® 40 Kuredux® Water and Carbon Glycolic acid (Polyglycolic acid) Dioxide etc. Biodegradation (%) Biodegradation 20 Hydrolytic decomposition Microbial degradation 0 0 10 20 30 Time (Days) * Tested under aerobic conditions at 136.4˚F in controlled compost Kuredux -Polyglycolic acid(PGA) - Hydrolyzability Kuredux® has the property of degradation on contact with water and its decomposition rate is higher than polylactic acid (PLA) which is also a degradable resin .