Physico-Chemical Analysis of Semi-Crystalline PEEK in Aliphatic and Aromatic Solvents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study of Polystyrene Degradation Using Continuous Distribution Kinetics in a Bubbling Reactor
Korean J. Chem. Eng., 19(2), 239-245 (2002) Study of Polystyrene Degradation Using Continuous Distribution Kinetics in a Bubbling Reactor Wang Seog Cha†, Sang Bum Kim* and Benjamin J. McCoy** School of Civil and Environmental Engineering, Kunsan National University, Kunsan 573-701, Korea *Department of Chemical Engineering, Korea University, Seoul 136-701, Korea **Department of Chemical Engineering and Material Science, University of California, Davis, CA 95616, USA (Received 3 April 2001 • accepted 10 September 2001) Abstract−A bubbling reactor for pyrolysis of a polystyrene melt stirred by bubbles of flowing nitrogen gas at at- mospheric pressure permits uniform-temperature distribution. Sweep-gas experiments at temperatures 340-370 oC allowed pyrolysis products to be collected separately as reactor residue(solidified polystyrene melt), condensed vapor, and uncondensed gas products. Molecular-weight distributions (MWDs) were determined by gel permeation chro- matography that indicated random and chain scission. The mathematical model accounts for the mass transfer of vaporized products from the polymer melt to gas bubbles. The driving force for mass transfer is the interphase differ- ence of MWDs based on equilibrium at the vapor-liquid interface. The activation energy and pre-exponential of chain scission were determined to be 49 kcal/mol and 8.94×1013 s−1, respectively. Key words: Pyrolysis, Molecular-Weight-Distribution, Random Scission, Chain-end Scission, Continuous Distribution Theory INTRODUCTION Westerout et al. proposed a random-chain dissociation (RCD) model, based on evaporation of depolymerization products less than Fundamental and practical studies of plastics processing, such a certain chain length. In a subsequent paper [Westerout et al., 1997b] as energy recovery (production of fuel) and tertiary recycle (recov- on screen-heater pyrolysis, the RCD model was further described. -

Pathways for Degradation of Plastic Polymers Floating in the Marine Environment
Environmental Science Processes & Impacts View Article Online CRITICAL REVIEW View Journal | View Issue Pathways for degradation of plastic polymers floating in the marine environment Cite this: Environ. Sci.: Processes Impacts ,2015,17,1513 Berit Gewert, Merle M. Plassmann and Matthew MacLeod* Each year vast amounts of plastic are produced worldwide. When released to the environment, plastics accumulate, and plastic debris in the world's oceans is of particular environmental concern. More than 60% of all floating debris in the oceans is plastic and amounts are increasing each year. Plastic polymers in the marine environment are exposed to sunlight, oxidants and physical stress, and over time they weather and degrade. The degradation processes and products must be understood to detect and evaluate potential environmental hazards. Some attention has been drawn to additives and persistent organic pollutants that sorb to the plastic surface, but so far the chemicals generated by degradation of the plastic polymers themselves have not been well studied from an environmental perspective. In this paper we review available information about the degradation pathways and chemicals that are formed by degradation of the six plastic types that are most widely used in Europe. We extrapolate that information Creative Commons Attribution 3.0 Unported Licence. to likely pathways and possible degradation products under environmental conditions found on the oceans' surface. The potential degradation pathways and products depend on the polymer type. UV-radiation and oxygen are the most important factors that initiate degradation of polymers with a carbon–carbon backbone, leading to chain scission. Smaller polymer fragments formed by chain scission are more susceptible to biodegradation and therefore abiotic degradation is expected to Received 30th April 2015 precede biodegradation. -

Biodeterioration of Untreated Polypropylene Microplastic Particles by Antarctic Bacteria
polymers Article Biodeterioration of Untreated Polypropylene Microplastic Particles by Antarctic Bacteria Syahir Habib 1, Anastasia Iruthayam 1, Mohd Yunus Abd Shukor 1 , Siti Aisyah Alias 2,3 , Jerzy Smykla 4 and Nur Adeela Yasid 1,* 1 Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] (S.H.); [email protected] (A.I.); [email protected] (M.Y.A.S.) 2 Institute of Ocean and Earth Sciences, C308 Institute of Postgraduate Studies, University of Malaya, Kuala Lumpur 50603, Malaysia; [email protected] 3 National Antarctic Research Centre, B303 Level 3, Block B, IPS Building, Universiti Malaya, Kuala Lumpur 50603, Malaysia 4 Institute of Nature Conservation, Polish Academy of Sciences, Mickiewicza, 33, 31-120 Kraków, Poland; [email protected] * Correspondence: [email protected]; Tel.: +60-(0)3-9769-8297 Received: 7 September 2020; Accepted: 22 October 2020; Published: 6 November 2020 Abstract: Microplastic pollution is globally recognised as a serious environmental threat due to its ubiquitous presence related primarily to improper dumping of plastic wastes. While most studies have focused on microplastic contamination in the marine ecosystem, microplastic pollution in the soil environment is generally little understood and often overlooked. The presence of microplastics affects the soil ecosystem by disrupting the soil fertility and quality, degrading the food web, and subsequently influencing both food security and human health. This study evaluates the growth and biodegradation potential of the Antarctic soil bacteria Pseudomonas sp. ADL15 and Rhodococcus sp. ADL36 on the polypropylene (PP) microplastics in Bushnell Haas (BH) medium for 40 days. -

Table 1. SOME NAMES and ABBREVIATIONS of Plastics and Elastomers
TABlE 1. SOME NAMES AND ABBREVIATIONS OF PlASTICS AND ElASTOMERS. Common name Abbreviation Acetal (homopolymer and copolymer) POM-H and POM-K Acrylate styrene acrylonitrile ASAorAAS Acrylate modified styrene acrylonitrile ASAorAAS Acrylic acid ester rubber ACM Acrylonitrile butadiene rubber or nitrile butadiene rubber NBR Acrylonitrile butadiene styrene ABS Acrylonitrile styrene/chlorinated polyethylene ACS Acrylonitrile methyl methacrylate AMMA Acrylonitrile styrene/EPR rubber or, acrylonitrile ethylene propylene styrene AES Alpha methyl styrene AMS Atactic polypropylene APPorPP-A Butadiene rubber or, cis-1,4-polybutadiene rubber or, polybutadiene rubber BR Butadiene styrene block copolymer BDS Butyl rubber IIR Bulk molding compound BMC Casein formaldehyde CF Cellulose acetate CA Cellulose acetate butyrate CAB Cellulose acetate propionate CAP Cellulose nitrate CN Chlorinated polyethylene CPEorCM Chlorinated polyvinyl chloride CPVC or, PVC-C Chloro-polyethylene or, chlorinated polyethylene. CM or CPE or, PE-C Chloroprene rubber or, polychloroprene rubber CR Chlorotrifluoroethylene ethylene copolymers ECfFE Cis-polyisoprene or, cis-1,4-polyisoprene IR Coumarone indene resins CIR Diallyl phthalate DAP Diallyl isophthalate DAIP Dough molding compound DMC Elastomeric alloy melt processable rubber EA-MPR Elastomeric alloy thermoplastic vulcanizate EA-TPV Epichlohydrin rubber CHR Epoxy or, epoxide EP Epoxy or, epoxide, with glass fiber EPGF Ethyl cellulose EC Ethylene acryic acid EAA Ethylene propylene diene monomer (an EPR terpolymer) EPDM -

Synthetic Polymeric Materials for Bone Replacement
Review Synthetic Polymeric Materials for Bone Replacement Mônica Rufino Senra and Maria de Fátima Vieira Marques * Technology Center, Bloco J, Instituto de Macromoleculas Professora Eloisa Mano, Universidade Federal do Rio de Janeiro, IMA-UFRJ, Av. Horacio Macedo, 2030, Rio de Janeiro RJ 21941-598, Brazil; [email protected] * Correspondence: [email protected] Received: 17 November 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: Some treatment options available to repair bone defects are the use of autogenous and allogeneic bone grafts. The drawback of the first one is the donor site’s limitation and the need for a second operation on the same patient. In the allograft method, the problems are associated with transmitted diseases and high susceptibility to rejection. As an alternative to biological grafts, polymers can be used in bone repair. Some polymers used in the orthopedic field are poly(methyl methacrylate), poly(ether-ether-ketone), and ultra-high molecular weight polyethylene (UHMWPE). UHMWPE has drawn much attention since it combines low friction coefficient and high wear and impact resistance. However, UHMWPE is a bioinert material, which means that it does not interact with the bone tissue. UHMWPE composites and nanocomposites with hydroxyapatite (HA) are widely studied in the literature to mitigate these issues. HA is the main component of the inorganic phase in the natural bone, and the addition of this bioactive filler to the polymeric matrix aims to mimic bone composition. This brief review discusses some polymers used in orthopedic applications, focusing on the UHMWPE/HA composites as a potential bone substitute. Keywords: poly(methyl methacrylate); poly(ether-ether-ketone); ultra-high molecular weight polyethylene; hydroxyapatite; orthopedic applications 1. -

Degradation and Recyclability of Poly (Ethylene Terephthalate)
Chapter 4 Degradation and Recyclability of Poly (Ethylene Terephthalate) S. Venkatachalam, Shilpa G. Nayak, Jayprakash V. Labde, Prashant R. Gharal, Krishna Rao and Anil K. Kelkar Additional information is available at the end of the chapter http://dx.doi.org/10.5772/48612 1. Introduction The physical and chemical properties of polymers depend on the nature, arrangement of chemical groups of their composition and the magnitude of intra or intermolecular forces i.e primary and secondary valence bonds present in the polymer. Degradation process occurs due to the influence of thermal, chemical, mechanical, radiative and biochemical factors occurring over a period of time resulting in deterioration of mechanical properties and colour of polymers. The degradation occurs due to changes accompanying with the main backbone or side groups of the polymer. Degradation is a chemical process which affects not only the chemical composition of the polymer but also the physical parameters such as colour of the polymer, chain conformation, molecular weight, molecular weight distribution, crystallinity, chain flexibility, cross-linking and branching. The nature of weak links and end groups in the polymers contribute to stability of polymers. The degradation process is initiated at the terminal units with subsequent depolymerization. For example paraformaldehyde with hydroxyl terminal starts to degrade at about 170°C whereas the same polymer with acetyl terminals decomposes at about 200°C [1]. Replacement of carbon main chain with hetero atoms like P, N, B increases the thermal stability e.g. PON polymers containing phosphorus, oxygen, nitrogen and silicon. The exposure of polymeric materials to environmental factors over a period of time will lead to deterioration of physical, chemical, thermal and electrical properties. -

Effect of Polymer Degradation on Polymer Flooding in Heterogeneous Reservoirs
polymers Article Effect of Polymer Degradation on Polymer Flooding in Heterogeneous Reservoirs Xiankang Xin 1 ID , Gaoming Yu 2,*, Zhangxin Chen 1,3, Keliu Wu 3 ID , Xiaohu Dong 1 and Zhouyuan Zhu 1 ID 1 College of Petroleum Engineering, China University of Petroleum, Beijing 102249, China; [email protected] (X.X.); [email protected] (Z.C.); [email protected] (X.D.); [email protected] (Z.Z.) 2 College of Petroleum Engineering, Yangtze University, Wuhan 430100, China 3 Department of Chemical and Petroleum Engineering, University of Calgary, Calgary, AB T2N 1N4, Canada; [email protected] * Correspondence: [email protected]; Tel.: +86-27-6911-1069 Received: 3 July 2018; Accepted: 31 July 2018; Published: 2 August 2018 Abstract: Polymer degradation is critical for polymer flooding because it can significantly influence the viscosity of a polymer solution, which is a dominant property for polymer enhanced oil recovery (EOR). In this work, physical experiments and numerical simulations were both used to study partially hydrolyzed polyacrylamide (HPAM) degradation and its effect on polymer flooding in heterogeneous reservoirs. First, physical experiments were conducted to determine basic physicochemical properties of the polymer, including viscosity and degradation. Notably, a novel polymer dynamic degradation experiment was recommended in the evaluation process. Then, a new mathematical model was proposed and an in-house three-dimensional (3D) two-phase polymer flooding simulator was designed to examine both polymer static and dynamic degradation. The designed simulator was validated by comparison with the simulation results obtained from commercial software and the results from the polymer flooding experiments. This simulator further investigated and validated polymer degradation and its effect. -

Mechanical Properties Thermoplastic Laminates of Polycarbonate
Scienc ile e & Singh et al., J Textile Sci Eng 2018, 8:4 xt e E T n : g DOI 10.4172/2165-8064.1000366 f i o n l e a e n r r i n u g o Journal of Textile Science & Engineering J ISSN: 2165-8064 Research Article Article OpenOpen Access Access Mechanical Properties Thermoplastic Laminates of Polycarbonate- Polyetherimide Blend with Glass Fibers Singh JC1*, Srivastava M2, Singh RK2 and Yadaw SB3 1Indian Institute of Technology, New Delhi, India 2Central Institute of Plastics Engineering and Technology, Hajipur, India 3Defence Material & Stores Research & Development Establishment, Kanpur, India Abstract Thermoplastic Laminates of Polycarbonate (PC)/Polyetherimide (PEI) blend with glass fibers were prepared by solution blending of PC & PEI in different ratio of 100/0, 95/5, 90/10, 85/15 and 80/20 using Dichloromethane as solvent followed by applying the resulting blend solutions over the cut sheet size (30 × 25 cm) of woven mat glass fibers & dried overnight, then stacked up 12 flies of the impregnates and consolidated in a hot press at 300ºC and 3-4 ton (30 kg/cm2) pressure for a duration of one hour & cooled. The laminates of PC/PEI blends/woven mat glass fibers were studied for their mechanical properties viz: Tensile, Flexural, Impact and Inter Laminar Shear Strength and thermo-mechanical properties viz: TGA, DSC, DMA as well as examination of morphology by SEM. The mechanical properties of laminates of PC/PEI blends/woven mat glass fibers shows an increasing trend in Inter Laminar Shear Strength, Tensile and Flexural Strength upto 20% PEI while Impact Strength shows an increase upto 10% and decreases while the percentage of PEI increases. -
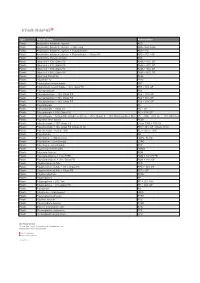
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

ASPHALTENE GRAFT COPOLYMER by FT-IR SPECTROSCOPY CT&F Ciencia, Tecnología Y Futuro, Vol
CT&F Ciencia, Tecnología y Futuro ISSN: 0122-5383 [email protected] ECOPETROL S.A. Colombia León-Bermúdez, Adan-Yovani; Salazar, Ramiro SYNTHESIS AND CHARACTERIZATION OF THE POLYSTYRENE - ASPHALTENE GRAFT COPOLYMER BY FT-IR SPECTROSCOPY CT&F Ciencia, Tecnología y Futuro, vol. 3, núm. 4, diciembre, 2008, pp. 157-167 ECOPETROL S.A. Bucaramanga, Colombia Available in: http://www.redalyc.org/articulo.oa?id=46530410 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SYNTHESIS AND CHARACTERIZATION OF THE POLYSTYRENE - ASPHALTENE GRAFT COPOLYMER BY FT-IR SPECTROSCOPY Ciencia, Tecnología y Futuro SYNTHESIS AND CHARACTERIZATION OF THE POLYSTYRENE - ASPHALTENE GRAFT COPOLYMER BY FT-IR SPECTROSCOPY Adan-Yovani León-Bermúdez1* and Ramiro Salazar1 1Universidad Industrial de Santander (UIS) - Grupo de Polímeros, Bucaramanga, Santander, Colombia e-mail: [email protected] (Received April 30, 2008; Accepted Nov. 27, 2008) he creation of new polymer compounds to be added to asphalt has drawn considerable attention because these substances have succeeded in modifying the asphalt rheologic characteristics and physical properties Tfor the enhancement of its behavior during the time of use. This work explains the synthesis of a new graft copolymer based on an asphalt fraction called asphaltene, modified with maleic anhydride. Polystyrene functionali- zation is conducted in a parallel fashion in order to obtain polybenzylamine resin with an amine – NH2 free group, that reacts with the anhydride graft groups in the asphaltene, thus obtaining the new Polystyrene/Asphaltene graft copolymer. -

Impact Strength of 3D Printed Polyether-Ether-Ketone (PEEK)
Solid Freeform Fabrication 2019: Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium – An Additive Manufacturing Conference Impact Strength of 3D Printed Polyether-ether-ketone (PEEK) Haijun Gong1,*, Xiaodong Xing2, Jan Nel1 1Department of Manufacturing Engineering, Georgia Southern University, Statesboro GA, USA 2College of Mechanical and Electrical Engineering, Harbin Engineering University, Harbin, China *Corresponding author ([email protected]) Abstract Polyether-ether-ketone (PEEK) is a high-performance thermoplastic with high heat-, high chemical-, high water-, and high wear-resistance. Its strength and durability also make it highly accepted for a range of industrial applications. 3D printing of PEEK filaments offers a unique quality and flexibility in making PEEK parts for low-volume production or special designs. This study investigates the impact strength of 3D printed PEEK materials. The specimens are fabricated using a fused deposition modeling (FDM) based 3D printer and tested by a pendulum impact tester in compliance with ASTM standard. The testing result is discussed with respect to the processing parameters and the annealing treatment. Impact strength comparison of PEEK materials manufactured by 3D printing and by conventional production is also conducted. Instruction Polyether-ether-ketone (PEEK) is a semi-crystalline polymer, which was developed in 1978. The PEEK is widely used as a high-performance thermoplastic due to its high heat-, high chemical-, high water-, high wear-resistance. Especially, PEEK and its varieties have been adopted for different biomedical treatment such as dental implants, orthopedics and maxillofacial surgery [1,2]. Its strength and durability also make it highly accepted for a range of industrial applications. For example, many automotive applications require to work at a temperature more than 120 °C. -

Predictive Lifetime Models of Polymer Insulation Degradation and Biorenewable Thermosets Through Cati
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2013 Polymer damage mitigation--predictive lifetime models of polymer insulation degradation and biorenewable thermosets through cationic polymerization for self-healing applications Peter Raymond Hondred Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Mechanics of Materials Commons Recommended Citation Hondred, Peter Raymond, "Polymer damage mitigation--predictive lifetime models of polymer insulation degradation and biorenewable thermosets through cationic polymerization for self-healing applications" (2013). Graduate Theses and Dissertations. 13105. https://lib.dr.iastate.edu/etd/13105 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Polymer damage mitigation—predictive lifetime models of polymer insulation degradation and biorenewable thermosets through cationic polymerization for self- healing applications by Peter Raymond Hondred A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Materials Science and Engineering Program of Study Committee: Michael R. Kessler, Major Professor Nicola