A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plastic Laws: Definitions
ELAW: Terms and Definitions from Plastic Laws Country Name of law if clear Link to law Term used Definition Estonia Waste Act https://www.riigiteataja.ee/en/eli/520012015021/consolideagricultural plastic means silage wrap film, silage covering film, tunnel film, net wrap, and plastic twine Australia, WA Environmental Protection (Plastichttps://www.slp.wa.gov.au/pco/prod/filestore.nsf/FileURL/mrdoc_41671.pdf/$FILE/Environmental%20Protection%20(Plastic%20Bags)%20Regulations%202018%20-%20%5B00-c0-00%5D.pdf?OpenElement Bags) Regulations 2018Barrier bag a plastic bag without handles used to carry unpackaged perishable food Environment Management (Container Deposit) Regulations Fiji 2011 https://files.elaw.org/app/index.do#storage/files/1/Shared/Documents/Legal/plastic/Laws_ByCountry/Fiji?pbeverage container means a jar, carton, can, bottle made of glass, polyethylene terephalate (PET) or aluminum that is or was sealed by its manufacturer External Policy: Environmental Levy on Plastic Bags Manufactured South Africa in South Africa https://www.sars.gov.za/AllDocs/OpsDocs/Policies/SE-PB-02%20-%20Environmental%20Levy%20on%20Plastic%20Bags%20Manufactured%20in%20South%20Africa%20-%20External%20Policy.pdfBin Liners A plastic bag used for lining a rubbish bin. Bahamas, The Environmental Protection (Control of Plastic Pollution)biodegradable Act, 2019 single-use plastic bag that is capable of being decomposed by bacteria or other living organisms Ville de Montreal By-Law 16- Canada, Montreal 051 http://ville.montreal.qc.ca/sel/sypre-consultation/afficherpdf?idDoc=27530&typeDoc=1biodegradable -

Microstructure and Properties of Polytetrafluoroethylene Composites
coatings Article Microstructure and Properties of Polytetrafluoroethylene Composites Modified by Carbon Materials and Aramid Fibers Fubao Zhang *, Jiaqiao Zhang , Yu Zhu, Xingxing Wang and Yuyang Jin School of Mechanical Engineering, Nantong University, Nantong 226019, China; [email protected] (J.Z.); [email protected] (Y.Z.); [email protected] (X.W.); [email protected] (Y.J.) * Correspondence: [email protected]; Tel.: +86-13646288919 Received: 12 October 2020; Accepted: 16 November 2020; Published: 18 November 2020 Abstract: Polytetrafluoroethylene (PTFE) is polymerized by tetrafluoroethylene, which has high corrosion resistance, self-lubrication and high temperature resistance. However, due to the large expansion coefficient, high temperature will gradually weaken the intermolecular bonding force of PTFE, which will lead to the enhancement of permeation absorption and the limitation of the application range of fluoroplastics. In order to improve the performance of PTFE, the modified polytetrafluoroethylene, filled by carbon materials and aramid fiber with different scales, is prepared through the compression and sintering. Moreover, the mechanical properties and wear resistance of the prepared composite materials are tested. In addition, the influence of different types of filler materials and contents on the properties of PTFE is studied. According to the experiment results, the addition of carbon fibers with different scales reduces the tensile and impact properties of the composite materials, but the elastic modulus and wear resistance are significantly improved. Among them, the wear rate of 7 µm carbon fiber modified PTFE has decreased by 70%, and the elastic modulus has increased by 70%. The addition of aramid fiber filler significantly reduces the tensile and impact properties of the composite, but its elastic modulus and wear resistance are significantly improved. -

Acrylic/PMMA Poly(Methyl Methacrylate) >
Product Information Sheet Acrylic/PMMA Poly(methyl methacrylate) Acrylic (PMMA) is a water clear transparent polymer suitable for indoor and outdoor applications. PMMA is an economical alternative to polycarbonate (PC) when extreme strength is not required. It also offers great UV stability, scratch resistance, and light transparency over PC. Conventus Polymers is an authorized distributor of LG MMA. Key Features 4Water clear and transparent 4Unsurpassed in ultraviolet resistance 4Excellent scratch resistance 4Highest light transmission 4Ease of molding 4Good chemical resistance 4Easy to color (transparent, translucent, and opaque) 4Excellent economical value Comparison of PMMA vs. Other Transparent Resins Scratch resistance Chemical Transmittance Heat Impact Weatherability Workability 92% resistance resistance 4H resistance 5 Superiority PMMA 4 PC 3 Transparent ABS SAN 2 PS 1 Note: PMMA is the only polymer family that offers the unique combination of UV resistance, light transmission, and scratch resistance. Applications 4 Lighting and lenses 4 Point of purchase displays 4 Optical applications 4 Automotive components 4 PPE equipment and sheet 4 Packaging and cosmetics 4 Appliance components 4 PMMA Product Information Sheet About Conventus Polymers Conventus Polymers offers distribution as well as superior custom compounding and masterbatch solutions in thermoplastic resins. Conventus partners with you to identify the best resins, reinforcements, additives, modifiers, and more to formulate a custom compound for your specific application requirements. Conventus delivers a combination of technology, performance, and quality with speed, flexibility, and service that is unparalleled in today’s industry. The goal is to work as your partner to make your vision a reality. Conventus Manufacturing Expertise The manufacturing facilities at Conventus excel with continuous improvement and highly developed process controls to ensure every batch produced meets our customer's wide array of specifications. -

Table 1. SOME NAMES and ABBREVIATIONS of Plastics and Elastomers
TABlE 1. SOME NAMES AND ABBREVIATIONS OF PlASTICS AND ElASTOMERS. Common name Abbreviation Acetal (homopolymer and copolymer) POM-H and POM-K Acrylate styrene acrylonitrile ASAorAAS Acrylate modified styrene acrylonitrile ASAorAAS Acrylic acid ester rubber ACM Acrylonitrile butadiene rubber or nitrile butadiene rubber NBR Acrylonitrile butadiene styrene ABS Acrylonitrile styrene/chlorinated polyethylene ACS Acrylonitrile methyl methacrylate AMMA Acrylonitrile styrene/EPR rubber or, acrylonitrile ethylene propylene styrene AES Alpha methyl styrene AMS Atactic polypropylene APPorPP-A Butadiene rubber or, cis-1,4-polybutadiene rubber or, polybutadiene rubber BR Butadiene styrene block copolymer BDS Butyl rubber IIR Bulk molding compound BMC Casein formaldehyde CF Cellulose acetate CA Cellulose acetate butyrate CAB Cellulose acetate propionate CAP Cellulose nitrate CN Chlorinated polyethylene CPEorCM Chlorinated polyvinyl chloride CPVC or, PVC-C Chloro-polyethylene or, chlorinated polyethylene. CM or CPE or, PE-C Chloroprene rubber or, polychloroprene rubber CR Chlorotrifluoroethylene ethylene copolymers ECfFE Cis-polyisoprene or, cis-1,4-polyisoprene IR Coumarone indene resins CIR Diallyl phthalate DAP Diallyl isophthalate DAIP Dough molding compound DMC Elastomeric alloy melt processable rubber EA-MPR Elastomeric alloy thermoplastic vulcanizate EA-TPV Epichlohydrin rubber CHR Epoxy or, epoxide EP Epoxy or, epoxide, with glass fiber EPGF Ethyl cellulose EC Ethylene acryic acid EAA Ethylene propylene diene monomer (an EPR terpolymer) EPDM -

Synthetic Polymeric Materials for Bone Replacement
Review Synthetic Polymeric Materials for Bone Replacement Mônica Rufino Senra and Maria de Fátima Vieira Marques * Technology Center, Bloco J, Instituto de Macromoleculas Professora Eloisa Mano, Universidade Federal do Rio de Janeiro, IMA-UFRJ, Av. Horacio Macedo, 2030, Rio de Janeiro RJ 21941-598, Brazil; [email protected] * Correspondence: [email protected] Received: 17 November 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: Some treatment options available to repair bone defects are the use of autogenous and allogeneic bone grafts. The drawback of the first one is the donor site’s limitation and the need for a second operation on the same patient. In the allograft method, the problems are associated with transmitted diseases and high susceptibility to rejection. As an alternative to biological grafts, polymers can be used in bone repair. Some polymers used in the orthopedic field are poly(methyl methacrylate), poly(ether-ether-ketone), and ultra-high molecular weight polyethylene (UHMWPE). UHMWPE has drawn much attention since it combines low friction coefficient and high wear and impact resistance. However, UHMWPE is a bioinert material, which means that it does not interact with the bone tissue. UHMWPE composites and nanocomposites with hydroxyapatite (HA) are widely studied in the literature to mitigate these issues. HA is the main component of the inorganic phase in the natural bone, and the addition of this bioactive filler to the polymeric matrix aims to mimic bone composition. This brief review discusses some polymers used in orthopedic applications, focusing on the UHMWPE/HA composites as a potential bone substitute. Keywords: poly(methyl methacrylate); poly(ether-ether-ketone); ultra-high molecular weight polyethylene; hydroxyapatite; orthopedic applications 1. -

Mechanical Properties Thermoplastic Laminates of Polycarbonate
Scienc ile e & Singh et al., J Textile Sci Eng 2018, 8:4 xt e E T n : g DOI 10.4172/2165-8064.1000366 f i o n l e a e n r r i n u g o Journal of Textile Science & Engineering J ISSN: 2165-8064 Research Article Article OpenOpen Access Access Mechanical Properties Thermoplastic Laminates of Polycarbonate- Polyetherimide Blend with Glass Fibers Singh JC1*, Srivastava M2, Singh RK2 and Yadaw SB3 1Indian Institute of Technology, New Delhi, India 2Central Institute of Plastics Engineering and Technology, Hajipur, India 3Defence Material & Stores Research & Development Establishment, Kanpur, India Abstract Thermoplastic Laminates of Polycarbonate (PC)/Polyetherimide (PEI) blend with glass fibers were prepared by solution blending of PC & PEI in different ratio of 100/0, 95/5, 90/10, 85/15 and 80/20 using Dichloromethane as solvent followed by applying the resulting blend solutions over the cut sheet size (30 × 25 cm) of woven mat glass fibers & dried overnight, then stacked up 12 flies of the impregnates and consolidated in a hot press at 300ºC and 3-4 ton (30 kg/cm2) pressure for a duration of one hour & cooled. The laminates of PC/PEI blends/woven mat glass fibers were studied for their mechanical properties viz: Tensile, Flexural, Impact and Inter Laminar Shear Strength and thermo-mechanical properties viz: TGA, DSC, DMA as well as examination of morphology by SEM. The mechanical properties of laminates of PC/PEI blends/woven mat glass fibers shows an increasing trend in Inter Laminar Shear Strength, Tensile and Flexural Strength upto 20% PEI while Impact Strength shows an increase upto 10% and decreases while the percentage of PEI increases. -
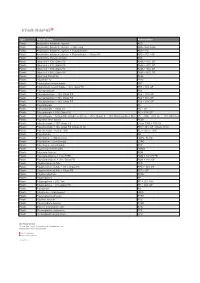
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Impact Strength of 3D Printed Polyether-Ether-Ketone (PEEK)
Solid Freeform Fabrication 2019: Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium – An Additive Manufacturing Conference Impact Strength of 3D Printed Polyether-ether-ketone (PEEK) Haijun Gong1,*, Xiaodong Xing2, Jan Nel1 1Department of Manufacturing Engineering, Georgia Southern University, Statesboro GA, USA 2College of Mechanical and Electrical Engineering, Harbin Engineering University, Harbin, China *Corresponding author ([email protected]) Abstract Polyether-ether-ketone (PEEK) is a high-performance thermoplastic with high heat-, high chemical-, high water-, and high wear-resistance. Its strength and durability also make it highly accepted for a range of industrial applications. 3D printing of PEEK filaments offers a unique quality and flexibility in making PEEK parts for low-volume production or special designs. This study investigates the impact strength of 3D printed PEEK materials. The specimens are fabricated using a fused deposition modeling (FDM) based 3D printer and tested by a pendulum impact tester in compliance with ASTM standard. The testing result is discussed with respect to the processing parameters and the annealing treatment. Impact strength comparison of PEEK materials manufactured by 3D printing and by conventional production is also conducted. Instruction Polyether-ether-ketone (PEEK) is a semi-crystalline polymer, which was developed in 1978. The PEEK is widely used as a high-performance thermoplastic due to its high heat-, high chemical-, high water-, high wear-resistance. Especially, PEEK and its varieties have been adopted for different biomedical treatment such as dental implants, orthopedics and maxillofacial surgery [1,2]. Its strength and durability also make it highly accepted for a range of industrial applications. For example, many automotive applications require to work at a temperature more than 120 °C. -

Synthesis and Process Optimization of Electrospun PEEK-Sulfonated Nanofibers by Response Surface Methodology
Materials 2015, 8, 4096-4117; doi:10.3390/ma8074096 OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Article Synthesis and Process Optimization of Electrospun PEEK-Sulfonated Nanofibers by Response Surface Methodology Carlo Boaretti, Martina Roso, Alessandra Lorenzetti and Michele Modesti * Department of Industrial Engineering, University of Padova, via Marzolo 9, 35131 Padova, Italy; E-Mails: [email protected] (C.B.); [email protected] (M.R.); [email protected] (A.L.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-049-827-5541; Fax: +39-049-827-5555. Academic Editor: Maryam Tabrizian Received: 22 May 2015 / Accepted: 30 June 2015 / Published: 7 July 2015 Abstract: In this study electrospun nanofibers of partially sulfonated polyether ether ketone have been produced as a preliminary step for a possible development of composite proton exchange membranes for fuel cells. Response surface methodology has been employed for the modelling and optimization of the electrospinning process, using a Box-Behnken design. The investigation, based on a second order polynomial model, has been focused on the analysis of the effect of both process (voltage, tip-to-collector distance, flow rate) and material (sulfonation degree) variables on the mean fiber diameter. The final model has been verified by a series of statistical tests on the residuals and validated by a comparison procedure of samples at different sulfonation degrees, realized according to optimized conditions, for the production of homogeneous thin nanofibers. Keywords: electrospinning; response surface methodology; sulfonated polyether ether ketone 1. Introduction Nanofibers are an interesting and versatile class of one-dimensional nanomaterials, with diameters ranging from tenths to hundreds of nanometers, which have been recognized as promising due to their outstanding properties in terms of high porosity, excellent pore interconnectivity, small diameters and high surface-to-volume ratio. -

Polymer Properties and Classification
Polymer Characterization LDPE Polyethylene low density HDPE Polyethylene high density ABS Acrylonitrile-butadiene-styrene SAN Styrene-acrylonitrile copolymer EVA Polyethylene co-vinyl acetate PVA Polyvinyl acetate PerkinElmer Solutions for Polymer Characterization Tg(ºC): -130 to 100 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): -130 to 100 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): 110 to 125 CpJ/(g*K): 1,25 to 1,7 Tg(ºC): 95 to 110 CpJ/(g*K): 1,2 Tg(ºC): -45 to 20 CpJ/(g*K): 2,3 Tg(ºC): 25 to 35 CpJ/(g*K): - Tm(ºC): 100 to 120 DHf (J/g): - Tm(ºC): 130 to 140 DHf (J/g): 293 Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): 30 to 100 DHf (J/g): 10 to 100 Tm( ºC ): - DHf (J/g): - Td(ºC): 490 to 500 Td(ºC): 490 to 500 Td(ºC): 420 Td(ºC): 420 Td(ºC): 480 Td(ºC): - PP Polypropylene PS PMMA Polymethylmethacrylate PBMA CA Polystyrene Polybuthylmethacrylate Cellulose acetate EP Epoxy resin Molecular Spectroscopy FTIR Differential Scanning Calorimetry Tg(ºC): -20 to -5 CpJ/(g*K): 1,8 Tg(ºC): 90 to 110 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): 85 to 100 CpJ/(g*K): 1,45 to 1,5 Tg(ºC): 15 to 25 CpJ/(g*K): - Tg(ºC): 45 to 60 CpJ/(g*K): - Tg(ºC): 50 to 200 CpJ/(g*K): 1,6 to 2,1 Identify and quantitate organic molecules and compounds, Glass transition & melting temperatures, crystallinity, heat of Understand chemical & physical composition of laminates & fusion, reaction rates, specific heat & heat capacity, curing, Tm(ºC): 165 to 175 DHf (J/g): 207 Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm( ºC): - DHf (J/g): - adhesives , Troubleshoot -

Acrylic Based Thermoplastic Elastomers: Design and Synthesis for Improved Mechanical Performance
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2017 All Acrylic Based Thermoplastic Elastomers: Design and Synthesis for Improved Mechanical Performance Wei Lu University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Materials Chemistry Commons, Organic Chemistry Commons, Polymer and Organic Materials Commons, Polymer Chemistry Commons, and the Polymer Science Commons Recommended Citation Lu, Wei, "All Acrylic Based Thermoplastic Elastomers: Design and Synthesis for Improved Mechanical Performance. " PhD diss., University of Tennessee, 2017. https://trace.tennessee.edu/utk_graddiss/4411 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Wei Lu entitled "All Acrylic Based Thermoplastic Elastomers: Design and Synthesis for Improved Mechanical Performance." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemistry. Jimmy W. Mays, Major Professor We have read this dissertation and recommend its acceptance: Alexei P. Sokolov, Michael D. Best, Thomas A. Zawodzinski Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) All Acrylic Based Thermoplastic Elastomers: Design and Synthesis for Improved Mechanical Performance A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Wei Lu May 2017 Copyright © 2017 by Wei Lu All rights reserved. -

Dielectric Spectroscopy and Thermally Stimulated Discharge Current in PEEK Film
Volume 28 (2003) número 2 www.scielo.br/eq Dielectric spectroscopy and thermally stimulated discharge current in PEEK film W. K. Sakamoto Departamento de Física e Química – FEG/UNESP - CEP 15385-000 Ilha Solteira –SP – Brasil Abstract: The complex permittivity of films of polyether ether ketone (PEEK) has been investigated over a wide range of frequency. There is no relaxation peak in the range of 1Hz to 105 Hz but in the low- frequency side (10-4 Hz) there is an evidence of a peak that also can be observed by thermally stimulated discharge current measurements. That peak is related with the glass transition temperature (Tg) of the polymer. The activation energy of the relaxation was found to be 0.44 eV, similar to that of several synthetic polymers. Space charges are important in the conduction mechanism as shown by discharging transient. Keywords: dielectric loss; thermally stimulated discharge current; polymer; activation energy; glass transition temperature. Introduction Although PEEK has already been used as insulating material and its electrical and dielectric Polyether ether ketone (PEEK) is an properties have been reported [4, 5], this paper aromatic polyether. It has been developed by I. C. reports results of dielectric spectroscopy in the wide I. Petrochemicals and Plastic Division as a high- frequency range of 10-5 Hz to 105 Hz and the nature temperature engineering thermoplastic [1]. of the thermally stimulated discharge current Because of the symmetry of the benzene rings (TSDC) in the temperature range from 10 oC to along the polymer backbone, PEEK offers above glass transition temperature (Tg) is analyzed.