09 September 2021 Aperto
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Editoriale Il Sindaco
Periodico di informazione a cura dell’Amministrazione Comunale - Registro Stampa del Tribunale di Asti n. 5/2011 Numero 5 - Dicembre 2013 - Direttore Responsabile Marinella Ferrero - Progetto Editoriale Marco Ferrante - Editore Comune di Piovà Massaia, Piazza Marconi n. 1 - 14026 Piovà Massaia (AT) www.comune.piovamassaia.at.it EDITORIALE arissimi piovatesi, eccoci finalmente con un nuovo Cnumero del nostro gior- nalino. Abbiamo tardato un po’ la pubblicazione perché purtroppo siamo stati costretti a diradare le uscite a causa dei costi di stampa e della necessità di economizza- re il più possibile. Nonostante il grande numero di iniziative svolte della G.R. n° 38 – 16335 del 29 giu- li, regionali e sanitarie, nell’agosto nei mesi scorsi, credo, comun- gno 1992 e n° 41 – 42433 del 9 gen- 2003, la popolazione stessa decise que, di aver inserito all’interno di naio 1995, purtroppo, però, i locali di istituire un Comitato, formato queste pagine tutte le maggiori e l’assistenza socio-sanitaria della da cinque persone scelte diretta- informazioni relative al nostro Casa di Riposo non potevano più mente dai cittadini piovatesi, più paese e il resoconto delle svariate dirsi a norma di legge e pertanto, un membro di diritto, che s’iden- attività tenutesi, per cui vi auguro dopo varie sollecitazioni dell’ASL, tificava nella persona del parroco, una buona lettura. la struttura fu costretta a chiude- allora Don Bartolomeo Novarese. Marinella Ferrero re. In apparenza, infatti, essa poteva I compiti di questo Comitato erano considerarsi in discreto stato, ma, quelli di valutare la possibilità di ria- trattandosi di un edificio di antica prire la Casa di Riposo e, in caso di IL S INDACO costruzione i locali non possedeva- esito positivo, predisporre un pro- no tutte le caratteristiche previste getto di ristrutturazione e trovare i n seguito a numerose richieste da dalla nuova legge e anche il tipo finanziamenti per attivarlo. -

Art and Exhibitions
ART AND EXHIBITIONS ASTI – PALAZZO MAZZETTI th Until July 15 ALEPPO. COME E’ STATA UCCISA UNA CITTA The exhibition curated by the journalist Domenico Quirico is dedicated to the drama of the inhabitants of Aleppo from 2011 to 2016. Visitors, while walking through city spaces recreated with multimedia installations, will meet the student, the sniper and the migrant. Guided tour on July 7 th at 5pm. th Until July 15 ALIGHIERO BOETTI. PERFILOEPERSEGNO th Alighiero Boetti is one of the most important and creative authors of the 20 century. The theme of the exhibition is inspired by the artist's phrase: “the pen represents for Westerners what the embroidery represents for Afghans: an over-individual memory containing parts of the collective biography”. The aim is to examine the relationship between the West and the East thanks to written and embroidery works. Opening times: Tue-Sun 10am-7pm (last admission at 6pm) Info: www.palazzomazzetti.it ASTI – FONDAZIONE GUGLIELMINETTI MUSEO EUGENIO GUGLIELMINETTI Summer break for exhibitions but not for the visits to the Museum dedicated to Eugenio Guglielminetti, the great set designer and artist Opening times on: Saturdays from 4.30pm to 6.30pm and on Sundays from 3.30pm to 6.30pm; guided tours by the young people of the Eugenio Guglielminetti Foundation. Last admission at 5.30pm without reservation. Info: [email protected] ; www.comune.asti.it ASTI – MUSEO DEL PALIO Until March 31st 2019 IMAGO SANCTI. SAN SECONDO CUSTODE DI ASTI E DEL SUO PALIO At the Palio Museum, the exhibition entitled "Imago Sancti. San Secondo Custode di Asti e del suo Palio” on the iconography of the Saint Patron from the 13 th century to the present day. -

Comune Di San Damiano D'asti (AT)
COMUNE DI SAN DAMIANO D’ASTI Provincia di Asti San Damiano d’Asti – Piazza Libertà n. 2 – CAP 14015 – tel. 0141/97.50.56 p.i./CF: 00086030053 – sito internet: www.comune.sandamiano.at.it E-mail: [email protected] ; PEC: [email protected] BANDO L’ASSEGNAZIONE IN LOCAZIONE DI ALLOGGI DI EDILIZIA SOCIALE CHE SI RENDERANNO DISPONIBILI NEL COMUNE DI SAN DAMIANO D’ASTI – L.R. 3/2010 E REGOLAMENTI ATTUATIVI APPROVATI CON D.P.R.G. 4 OTTOBRE 2011 Ai sensi e per gli effetti della Legge Regionale 17 febbraio 2010, n. 3 e s.m.i., ad oggetto: “Norme in materia di edilizia sociale” e dei regolamenti attuativi approvati con D.P.R.G. 4 ottobre 2011 e pubblicati sul B.U.R.P. n. 40s1 del 6.10.2011, ai sensi della Delibera della Giunta Comunale n. 2 del 29/01/2018, è indetto il bando generale per l’assegnazione in locazione di alloggi di edilizia sociale, in disponibilità al Comune di San Damiano d’Asti fatti salvi gli alloggi riservati per le particolari situazioni di emergenza abitativa di cui art. 10 della citata legge regionale, che si renderanno disponibili nel periodo di validità della graduatoria . Ai sensi dell’art. 11 della L. R. n. 3/2010 è inoltre disposta la formazione della graduatoria degli appartenenti alle forze dell’ordine e dei vigili del fuoco per l’assegnazione degli alloggi di edilizia sociale ad essi destinati. AMBITO TERRITORIALE DI RIFERIMENTO Ai sensi dell'art. 5, comma 2 della L.R. n. -

Gli Orari Delle Messe Prefestive E Festive in Diocesi
20 ______________________________________________________________________________________________________________ 5 aprile 2019 | Gazzetta d’Asti GLI ORARI DELLE MESSE PREFESTIVE E FESTIVE IN DIOCESI PARROCCHIE CITTADINE CATTEDRALE prefestiva 18 S. MARTINO domenica 10 domenica 10.30 • 12 • 18 (sospesa luglio e agosto) S. PAOLO prefestiva 17,30 domenica 8,30 • 10,30 • 11,45 • 17,30 MARIA AUSILIATRICE (VIATOSTO) domenica 11,15 S. PIETRO prefestiva 17 domenica 9 • 10,30 • 17 NOSTRA SIGNORA prefestiva 18 DI LOURDES domenica 9 • 11,15 • 18,30 (sospesa da giugno a S. SECONDO prefestiva 18 domenica 10 • 11,15 • 18 (TORRETTA) settembre) S. SILVESTRO prefestiva 17,30 domenica 8 S. CATERINA prefestiva 17 domenica 11 SACRO CUORE prefestiva 18 domenica 11,15 S. DOMENICO SAVIO prefestiva 17,30 domenica 8,30 • 10,30 • 17,30 SS. ANNUNZIATA prefestiva 18 domenica 8,30 • 11,15 S. GIOVANNI BOSCO prefestiva 18,30 domenica 8,45 • 10 • 11,30 • 18,30 (TANARO) CHIESE CITTADINE NON PARROCCHIALI BELLAVISTA domenica 9 S. ROCCO prefestiva 16,30 CAPPELLANIA prefestiva 18 domenica 10 TRINCERE domenica 9,30 S. GIUSEPPE MARELLO VALMANERA domenica 9,30 SANTUARI CITTADINI MADONNA DEL PORTONE prefestiva 17,30 domenica 10 • 17,30 S. GIUSEPPE prefestiva 17,30 (ora solare) - 18 (ora legale) domenica 9 • 21; 21,30 (da maggio ad agosto) CHIESE PARROCCHIALI EXTRA URBANE AGLIANO TERME prefestiva 17 (ora solare) - 18 (ora legale) CERRETO domenica 11,30 domenica 9,30-11 CERRO TANARO prefestiva 17,30 ALBUGNANO prefestiva 16 residenza anziani Il Giglio domenica 10 domenica 11 CHIUSANO prefestiva 18 (dopo Pasqua 21) ANTIGNANO prefestiva 18 CINAGLIO domenica 10 domenica 8,30 in alternanza (Gonella e Perosini) domenica 11,15 in parrocchia CISTERNA D’ASTI prefestiva 17 (ora solare) - 18 (ora legale); domenica 8,45 Valmellana • 9,45 San Matteo AZZANO prefestiva 16 (periodo estivo - Fraz. -

Codice A18060 D.D. 18 Giugno 2015, N. 1436 Autorizzazione Idraulica N
REGIONE PIEMONTE BU41 15/10/2015 Codice A18060 D.D. 18 giugno 2015, n. 1436 Autorizzazione idraulica n. 1497 per la realizzazione di tre attraversamenti in sub alveo del corso d'acqua pubblica denominato rio Gravina o Cravina, mediante condotta per acquedotto in prossimita' della S.P. n. 19, nel Comune di San Damiano d'Asti (AT) a confine con il Comune di San Martino Alfieri (AT). Richiedente: Acquedotto della Piana S.p.A. - Gestione Servizio Idrico Integrato con sede in Villanova d'Asti. Con nota n° 2498 in data 12/05/2015 (ns. prot. n° 26584 del 13/05/2015) il Legale Rappresentante della Società Acquedotto della Piana S.p.A. con sede in via Carlo V, n° 53 – Villanova d’Asti (AT), Codice Fiscale e Partita IVA 00099020059, ha presentato istanza per il rilascio di concessione demaniale per la realizzazione di numero tre attraversamenti in sub alveo del rio Gravina o Cravina, in Comune di San Damiano d’Asti ed al confine con il Comune di San Martino Alfieri, mediante condotta per l’alimentazione dell’Acquedotto delle Colline Alfieri nell’ambito del progetto di “Lavori di potenziamento e collegamento infrastrutture del servizio idrico integrato nell’area della Piana. Collegamento tra la rete acquedottistica del Comune di San Damiano d’Asti e quella a servizio dei Comuni di San Martino Alfieri, Antignano, Celle Enomondo e Revigliasco d’Asti”. Gli attraversamenti del rio Gravina o Cravina, progettati in sub alveo, saranno ubicati tutti in parallelo alla S.P. 19 e sono indicati con i numeri 1-2-3 negli elaborati tecnici e grafici allegati all’istanza: Attraversamento 1 : sarà realizzato da una tubazione in PEAD DN 280 mm PN 25 inserita in tubo camicia in acciaio bitumato DN 400; la tubazione in subalveo sarà protetta con bauletto di calcestruzzo armato CLS C25/30, avente dimensioni di 120 x 120 cm e collocato ad una idonea profondità che garantisce un franco minimo di 1,00 m tra l’estradosso del bauletto ed il fondo alveo del rio Cravina. -

Listino Dei Valori Immobiliari Dei Terreni Agricoli
ISSN: 2280-191X LISTINO DEI VALORI IMMOBILIARI DEI TERRENI AGRICOLI PROVINCIA DI ASTI LISTINO 2019 RILEVAZIONE ANNO 2018 quotazioni dei valori di mercato dei terreni agricoli entro un minimo e un massimo per le prin cipali colture in ciascun comune privati professionisti edizioni pubblica amministrazione OSSERVATORIO DEI VALORI AGRICOLI – PROVINCIA DI ASTI – RILEVAZIONE 2 0 1 8 Hanno collaborato alla formazione del listino GIOVANNI GRIFFA, ingegnere, attualmente libero professionista. È stato dirigente dell’Agenzia del territorio, tra l’altro direttore degli uffici provinciali di Asti ed Alessandria, direttore degli uffici regionali della Liguria , del Piemonte e della Valle d’Aosta. Oltre a svolgere numerosi incarichi presso l’Amministrazione Finanziaria, è stato più volte componente della Commissione Provinciale Espropri. Ha svolto incarichi professionali di consulenza estimale e tecnica presso diversi Enti Pubblici. È Consulente Tecnico d’Ufficio presso il Tribunale di Asti dal 1990. ANTONIO IOVINE, ingegnere libero professionista consulente in materia di catasto ed estimo, attualmente membro della Commissione Provinciale espropri di Roma. È stato dirigente dell’Agenzia del territorio, responsabile dell’Area per i servizi catastali della Direzione centrale cartografia, catasto e pubblicità immobiliare, membro della Commissione Censuaria Centrale. Autore/coautore di vari testi in materia di catasto, topografia ed estimo, ha svolto numerosi incarichi di docenza per formazione in materia di estimo, espropriazioni e catasto. La redazione gradisce indicazioni costruttive o suggerimenti migliorativi ([email protected]). Disclaimer L’elaborazione del testo, anche se curata con scrupolosa attenzione, non può comportare specifiche responsabilità per errori o inesattezze. Altresì, l’uso dei dati riportati nel listino presuppone una autonoma e preventiva condivisione degli stessi da parte dell’utilizzatore, con assunzione diretta di ogni responsabilità che ne dovesse derivare dall’uso medesimo. -

50 Ore Luogo Di Svolgimento: Alfaform, Via Gaeta 10
Titolo del corso: Tecniche di gestione siti web Durata: 50 ore Luogo di svolgimento: Alfaform, Via Gaeta 10 – 14100 Asti Periodo partenza previsto*: da settembre 2018 a gennaio 2019 Obiettivi: Il corso si pone l'obiettivo di fornire le conoscenze e le capacita' necessarie per: ● strutturare un sito internet su specifiche fornite; ● gestire alias, redirect e rewriting, configurare domini virtuali, utilizzare tecniche di storyboarding ed infine utilizzare il CSM Wordpress per la creazione e la gestione di siti web statici e dinamici; ● l'utilizzo di alcuni programmi di grafica digitale della Suite Adobe (Photoshop CS6, Illustrator CS6) livello base. Destinatari: Dipendenti e titolari di Piccole e Micro Imprese con sede operativa in uno dei seguenti comuni astigiani: Asti, Albugnano, Antignano, Baldichieri, Canelli, Castagnole delle Lanze, Castagnole Monferrato, Castell'Alfero, Castello di Annone, Chiusano d'Asti, Cisterna, Costigliole, Ferrere, Isola d'Asti, Monale, Moncalvo, Montegrosso, Montiglio, Nizza Monferrato, Portacomaro, Revigliasco, Rocchetta Tanaro, San Damiano d'Asti, San Martino Alfieri, Tigliole, Tonco, Vigliano, Villanova d'Asti, Villafranca. Programma: ACCOGLIENZA E PRINCIPI ORIZZONTALI: L'unita' formativa ha come obiettivo l'introduzione dell'allievo/a nell'agenzia formativa e nel gruppo classe, la conoscenza dei contenuti, delle tappe, delle finalita' del percorso formativo, delle modalita' di erogazione, nonche' del sistema di regole presente nell'agenzia. Inoltre nell’Unità Formativa verra’ introdotto l’argomento del POR FSE 2014-2020, tali principi verranno approfonditi e contestualizzati con esempi pratici ed interventi specifici durante la trattazione dei successivi contenuti professionalizzanti. GESTIONE DI SITI WEB: In questa Unita’ Formativa verranno affrontati gli argomenti inerenti la gestione di un sito web e nello specifico, le tecniche di configurazione di un server, la normativa in materia di privacy e di sicurezza dei dati, l'utilizzo del software di sviluppo web. -

Valori Agricoli Medi Della Provincia Annualità 2018
Ufficio del territorio di ASTI Data: 26/11/2019 Ora: 11.31.34 Valori Agricoli Medi della provincia Annualità 2018 Dati Pronunciamento Commissione Provinciale Pubblicazione sul BUR n. del n. del REGIONE AGRARIA N°: 1 REGIONE AGRARIA N°: 2 COLLINE ALTO MONFERRATO ASTIGIANO MEDIO MONFERRATO ASTIGIANO Comuni di: ALBUGNANO, ARAMENGO, BERZANO DI SAN PIETRO, Comuni di: ANTIGNANO, ASTI, BALDICHIERI D`ASTI, CALLIANO BUTTIGLIERA D`ASTI, CANTARANA, CAPRIGLIO, CASTELNUOVO MONFERRATO, CAMERANO CASASCO, CASORZO, CASTAGNOLE DON BOSCO, CELLARENGO, CERRETO D`ASTI, CISTERNA D`ASTI, MONFERRATO, CASTELL ALFERO, CASTELLERO, CELLE COCCONATO, CORTANDONE, CORTANZE, CORTAZZONE, DUSINO ENOMONDO, CHIUSANO D`ASTI, CINAGLIO, COLCAVAGNO, SAN MICHELE, FERRERE, MARETTO, MONALE, MONCUCCO CORSIONE, COSSOMBRATO, CUNICO, FRINCO, GRANA, GRAZZANO TORINESE, MONTAFIA, MONTIGLIO, MORANSENGO, PASSERANO BADOGLIO, MONCALVO, MONTECHIARO D`ASTI, MONTEMAGNO, MARMORITO, PIEA, PINO D`ASTI, PIOVA MASSAIA, ROATTO, PENANGO, PORTACOMARO, REVIGLIASCO D`ASTI, SAN DAMIANO ROBELLA, SAN PAOLO SOLBRITO, TONENGO, VALFENERA, VIALE, D`ASTI, SAN MARTINO ALFIERI, SCANDELUZZA, SCURZOLENGO, VILLAFRANCA D`ASTI, VILLANOVA D`ASTI, MONTIGLIO SETTIME, SOGLIO, TIGLIOLE D`ASTI, TONCO, VIARIGI, VILLA SAN MONFERRATO SECONDO COLTURA Valore Sup. > Coltura più Informazioni aggiuntive Valore Sup. > Coltura più Informazioni aggiuntive Agricolo 5% redditizia Agricolo 5% redditizia (Euro/Ha) (Euro/Ha) BOSCO CEDUO 2649,00 3152,00 BOSCO D`ALTO FUSTO 6617,00 8257,00 BOSCO MISTO 3468,00 3970,00 FRUTTETO 12356,00 23236,00 INCOLTO PRODUTTIVO 570,00 570,00 NOCCIOLETO 9174,00 11466,00 ORTO 43512,00 ORTO IRRIGUO 43512,00 PASCOLO 1010,00 1010,00 Pagina: 1 di 6 Ufficio del territorio di ASTI Data: 26/11/2019 Ora: 11.31.34 Valori Agricoli Medi della provincia Annualità 2018 Dati Pronunciamento Commissione Provinciale Pubblicazione sul BUR n. -

Commission Implementing Decision of 7 February 2019 on the Publication
C 60/4 EN Official Journal of the European Union 15.2.2019 COMMISSION IMPLEMENTING DECISION of 7 February 2019 on the publication in the Official Journal of the European Union of an application to amend the specification for a name in the wine sector in accordance with Article 105 of Regulation (EU) No 1308/2013 of the European Parliament and of the Council (Barbera d’Asti (PDO)) (2019/C 60/05) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 (1), and in particular Article 97(3) thereof, Whereas: (1) Italy submitted an application to amend the specification for the name ‘Barbera d’Asti’ in accordance with Article 105 of Regulation (EU) No 1308/2013. (2) The Commission examined the application and found that the conditions laid down in Articles 93 to 96, 97(1), 100, 101 and 102 of Regulation (EU) No 1308/2013 had been met. (3) In order to allow statements of objection to be submitted in accordance with Article 98 of Regulation (EU) No 1308/2013, the application to amend the specification for the name ‘Barbera d’Asti’ should be published in the Official Journal of the European Union, HAS DECIDED AS FOLLOWS: Sole Article The application to amend the specification for the name ‘Barbera d’Asti’ (PDO) in accordance with Article 105 of Regu lation (EU) No 1308/2013 is contained in the Annex to this Decision. -

Vineyard Landscape of Langhe-Roero and Monferrato (Italy)
1 Basic data Vineyard Landscape of Langhe-Roero Included in the Tentative List and Monferrato 1 June 2006 (Italy) International Assistance from the World Heritage No 1390 rev Fund for preparing the Nomination None Date received by the World Heritage Centre 21 January 2011 Official name as proposed by the State Party 30 January 2013 The Vineyard Landscape of Piedmont: Langhe-Roero and Monferrato Background This is a nomination whose examination has been Location deferred (36 COM, Saint Petersburg, 2012). The nominated serial property is located in the Piedmont region. It is comprised of six separate components in the The World Heritage Committee has adopted the following provinces of: decision (decision 36 COM 8B.32): Cuneo (properties n°1 Langa of Barolo, n°2 Grinzane Decision: 36 COM 8B.32 Cavour Castle, n°3 Hills of Barbaresco, and part of property n°5 Canelli and Asti Spumante), The World Heritage Committee, Asti (property n°4 Nizza Monferrato and Barbera, and 1 Having examined Documents WHC-12/36.COM/8B and part of property n°5 Canelli and Asti Spumante) WHC-12/36.COM/INF.8B1, Alessandria (property n°6 Monferrato of the Infernot) Italy 2 Defers the examination of the nomination of the Vineyard Landscape of Piedmont: Langhe-Roero and Monferrato, Brief description Italy, to the World Heritage List, in order to allow the State The vineyard landscapes of Langhe-Roero and Party, with the advice of ICOMOS and the World Heritage Monferrato in the Piedmont region cover five distinct Centre, if requested, to: winegrowing areas and one castle, whose names are a. -

Popolazione Dettaglio Comuni
SSERVATORIO DELLA CONGIUNTURA POPOLAZIONE PROVINCIA DI ASTI – Anno 2012 (Fonte:elaborazione dati ISTAT – Demo: demografia in cifre) Bilancio demografico della popolazione residente nei Comuni della provincia Popolazione Popolazione Saldo Saldo Comuni residente al Nati Morti Iscritti Cancellati residente al 31 naturale migratorio 1° gennaio dicembre Agliano 1678 12 31 -19 98 71 27 1686 Albugnano 540 2 11 -9 32 35 -3 528 Antignano 1020 5 22 -17 37 32 5 1008 Aramengo 622 7 6 1 32 23 9 632 Asti 73863 707 838 -131 2554 1966 588 74320 Azzano d'Asti 417 5 2 3 19 18 1 421 Baldichieri d'Asti 1105 8 9 -1 92 71 21 1125 Belveglio 332 4 5 -1 31 10 21 352 Berzano di San Pietro 427 2 5 -3 25 29 -4 420 Bruno 351 0 5 -5 10 16 -6 340 Bubbio 912 5 19 -14 50 32 18 916 Buttigliera d'Asti 2563 27 26 1 166 99 67 2631 Calamandrana 1776 10 20 -10 74 75 -1 1765 Calliano 1391 5 25 -20 50 61 -11 1360 Calosso 1322 2 20 -18 66 53 13 1317 Camerano Casasco 477 5 9 -4 17 12 5 478 Canelli 10560 80 147 -67 375 288 87 10580 Cantarana 1019 6 15 -9 43 51 -8 1002 Capriglio 299 1 8 -7 16 20 -4 288 Casorzo 654 4 14 -10 35 19 16 660 Cassinasco 591 2 12 -10 35 33 2 583 Provincia di Asti - Anno 2012 SSERVATORIO DELLA CONGIUNTURA POPOLAZIONE PROVINCIA DI ASTI – Anno 2012 (Fonte:elaborazione dati ISTAT – Demo: demografia in cifre) Bilancio demografico della popolazione residente nei Comuni della provincia Popolazione Popolazione Saldo Saldo Comuni residente al Nati Morti Iscritti Cancellati residente al 31 naturale migratorio 1° gennaio dicembre Castagnole delle Lanze 3786 27 50 -
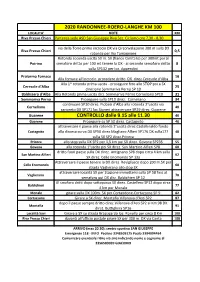
2020 RANDONNEE-ROERO-LANGHE KM 100 LOCALITA' NOTE KM Riva Presso Chieri Partenza Sede ASD San Giuseppe Riva Sez
2020 RANDONNEE-ROERO-LANGHE KM 100 LOCALITA' NOTE KM Riva Presso Chieri Partenza sede ASD San Giuseppe Riva Sez. Ciclismo ore 7,30 - 8,30 via della Torre primo incrocio DX via Circonvalazione 300 m sulla DX Riva Presso Chieri 0,5 rotonda per Via Tamagnone Rotonda seconda uscita 50 m. SX (fianco Cimitero) per 300mt poi al Poirino semaforo dritto per 100 mt tenere la DX - al secondo semaforo dritto 8 sulla SP132 per loc. Appendini Pralormo Fornace 16 Alla fornace all'incrocio procedere dritto DX direz.Ceresole d'Alba Alla 1^ rotonda prima uscita - proseguire fino allo STOP poi a SX Ceresole d'Alba 25 direzione Sommariva Perno SP 10 Baldissero d'Alba Alla Rotonda prima uscita dirz. Sommariva Perno Corneliano SP10 31 Sommariva Perno Proseguire sulla SP10 direz. Corneliano 34 continuare SP10 direz. Piobesi d'Alba alla rotonda 2°uscita via Corneliano 40 canoreto DX SP171 loc.Sioneri attraversare SR29 direz. Guarene Guarene CONTROLLO dalle 9.15 alle 11.30 46 Guarene Proseguire su SP 50 direz. Castagnito 46 attraversare il paese alla rotonda 2°uscita direz.Castellinaldo fondo Castagnito alla discesa curva DX SP50 direz.Magliano Alfieri SP176 DX sulla177 48 sulla SX SP2 direz.Priocca Priocca allo stop sulla DX SP2 per 1,5 km poi SX direz. Govone SP235 55 Govone alla rotonda 1°uscita poi SX direz. San Martino Alfieri SP8 60 dritto fuori paese sulla DX direz. Antignano SP8 dopo circa 4 km sulla San Martino Alfieri 62 SX direz. Celle Enomondo SP 13a Attraversare il paese tenere la DX direz. Revigliasco dopo 200 m.SX per Celle Enomondo 68 strada Vaglierano allo stop SX attraversare località SX per Stazione immettersi sulla SP 58 fino al Vaglierano 70 semaforo poi DX dirz.