Guide to Services 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zoology Addition to the Mite Fauna in Human Habitation from South
Volume : 5 | Issue : 7 | July 2016 • ISSN No 2277 - 8179 | IF : 3.508 | IC Value : 69.48 Original Research Paper Original Research Paper Volume : 5 | Issue : 7 | July 2016 • ISSN No 2277 - 8179 | IF : 3.508 | IC Value : 69.48 Zoology Addition To The Mite Fauna in Human KEYWORDS : Human habitation, Prostigmata, Mesostigmata, Astigmata, Habitation From South Bengal South Bengal Post Graduate Department of Zoology, Vidyasagar College, Salt Lake City, CL Ananya Das Block, Kolkata 700 091 Post Graduate Department of Zoology, Vidyasagar College, Salt Lake City, CL S.K. Gupta Block, Kolkata 700 091 Post Graduate Department of Zoology, Vidyasagar College, Salt Lake City, CL N. Debnath Block, Kolkata 700 091 ABSTRACT The present paper reports the occurrence of 111 species of mites belonging to 69 genera,27 families under 3 orders collected from a total of 40 samples representing 5 different habitats viz. stored products, house dust, bird nests, cattle sheds and roof gardens from 5 districts of South Bengal. Among the 5 habitats, cattle shed provided richest diversity both in respect of species and genera followed by stored product habitat and the minimum was bird nest which represented only 11 species. The family level diversity was also highest in case of cattle sheds followed by stored products and the minimum was in roof garden. There was not a single species which could be collected from all the 5 habitats though; of course, there was 1 species which represented 4 out of 5 habitats. Therefore, cattle sheds proved to be habitat showing highest diversity. The order Prostigmata represented highest number of species followed by Astigmata. -

Arthropod Parasites in Domestic Animals
ARTHROPOD PARASITES IN DOMESTIC ANIMALS Abbreviations KINGDOM PHYLUM CLASS ORDER CODE Metazoa Arthropoda Insecta Siphonaptera INS:Sip Mallophaga INS:Mal Anoplura INS:Ano Diptera INS:Dip Arachnida Ixodida ARA:Ixo Mesostigmata ARA:Mes Prostigmata ARA:Pro Astigmata ARA:Ast Crustacea Pentastomata CRU:Pen References Ashford, R.W. & Crewe, W. 2003. The parasites of Homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Taylor & Francis. Taylor, M.A., Coop, R.L. & Wall, R.L. 2007. Veterinary Parasitology. 3rd edition, Blackwell Pub. HOST-PARASITE CHECKLIST Class: MAMMALIA [mammals] Subclass: EUTHERIA [placental mammals] Order: PRIMATES [prosimians and simians] Suborder: SIMIAE [monkeys, apes, man] Family: HOMINIDAE [man] Homo sapiens Linnaeus, 1758 [man] ARA:Ast Sarcoptes bovis, ectoparasite (‘milker’s itch’)(mange mite) ARA:Ast Sarcoptes equi, ectoparasite (‘cavalryman’s itch’)(mange mite) ARA:Ast Sarcoptes scabiei, skin (mange mite) ARA:Ixo Ixodes cornuatus, ectoparasite (scrub tick) ARA:Ixo Ixodes holocyclus, ectoparasite (scrub tick, paralysis tick) ARA:Ixo Ornithodoros gurneyi, ectoparasite (kangaroo tick) ARA:Pro Cheyletiella blakei, ectoparasite (mite) ARA:Pro Cheyletiella parasitivorax, ectoparasite (rabbit fur mite) ARA:Pro Demodex brevis, sebacceous glands (mange mite) ARA:Pro Demodex folliculorum, hair follicles (mange mite) ARA:Pro Trombicula sarcina, ectoparasite (black soil itch mite) INS:Ano Pediculus capitis, ectoparasite (head louse) INS:Ano Pediculus humanus, ectoparasite (body -

Demodex Folliculorum in Nasal Discharge: a Case Report of Yet Unknown Significance
Global Journal of Otolaryngology ISSN 2474-7556 Case Report Glob J Otolaryngol Volume 18 Issue 2 - November 2018 Copyright © All rights are reserved by Neelam Riyaz Attar DOI: 10.19080/GJO.2018.18.555984 Demodex Folliculorum In Nasal Discharge: A Case Report of Yet Unknown Significance Neelam R Attar* Department of Microbiology, Assistant Professor, India Submission: November 15, 2018; Published: November 26, 2018 *Corresponding author: Neelam Riyaz Attar, Department of Microbiology, Assistant Professor, Niyaz Manzil, Kolhapur, India Abstract Demodex mites are the parasites residing in the pilo-sebaceous follicle and sebaceous gland. They are frequently isolated from cases of Demodexfolliculitis, folliculorum. Rosacea and various other inflammatory dermatoses. We report a possible case of demodecosis in patient of mucormycosis. Nasal scraping and discharge was negative for fungal elements but contained high density of gravid Demodex mites. The species was identified as Keywords: Demodex mites; Demodecosis; Nasal scrapping; Diabetes mellitus Introduction on SDA agar and incubated at 37C and 25C for 4 weeks. The Demodex mites are the normal inhabitants of pilosebaceous culture was reported as negative. Diagnosis of mucormycosis unit and gland. Demodex folliculorum and Demodex brevis are the two species found all over the body especially areas dense presence of broad non septate hyphae with right angle branching. in sebaceous glands including face, neck, back and chest [1- was confirmed by biopsy of right middle meatus which showed 3]. Previously thought to be harmless commensal they are now as it was longer than its counterpart D. brevis (Size 0.1 – 0.2 recently implicated as the causative agent of many dermatoses Demodex species was identified as that of Demodex folliculorum mm) [6]. -

Deconstructing Canine Demodicosis”
TESIS DOCTORAL TITULO: “Deconstructing canine demodicosis” AUTOR: Ivan Ravera DIRECTORES: Lluís Ferrer, Mar Bardagí, Laia Solano Gallego. PROGRAMA DE DOCTORADO: Medicina i Sanitat Animals DEPARTAMENTO: Medicina i Cirurgia Animals UNIVERSIDAD: Universitat Autònoma de Barcelona 2015 Dr. Lluis Ferrer i Caubet, Dra. Mar Bardagí i Ametlla y Dra. Laia María Solano Gallego, docentes del Departamento de Medicina y Cirugía Animales de la Universidad Autónoma de Barcelona, HACEN CONSTAR: Que la memoria titulada “Deconstructing canine demodicosis” presentada por el licenciado Ivan Ravera para optar al título de Doctor por la Universidad Autónoma de Barcelona, se ha realizado bajo nuestra dirección, y considerada terminada, autorizo su presentación para que pueda ser juzgada por el tribunal correspondiente. Y por tanto, para que conste firmo el presente escrito. Bellaterra, el 23 de Septiembre de 2015. Dr. Lluis Ferrer, Dra. Mar Bardagi, Ivan Ravera Dra. Laia Solano Gallego Directores de la tesis doctoral Doctorando AGRADECIMIENTOS A los alquimistas de guantes azules A los otros luchadores - Ester Blasco - Diana Ferreira - Lola Pérez - Isabel Casanova - Aida Neira - Gina Doria - Blanca Pérez - Marc Isidoro - Mercedes Márquez - Llorenç Grau - Anna Domènech - los internos del HCV-UAB - Elena García - los residentes del HCV-UAB - Neus Ferrer - Manuela Costa A los veterinarios - Sergio Villanueva - del HCV-UAB - Marta Carbonell - dermatólogos españoles - Mónica Roldán - Centre d’Atenció d’Animals de Companyia del Maresme A los sensacionales genetistas -

Arthropods of Public Health Significance in California
ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA California Department of Public Health Vector Control Technician Certification Training Manual Category C ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA Category C: Arthropods A Training Manual for Vector Control Technician’s Certification Examination Administered by the California Department of Health Services Edited by Richard P. Meyer, Ph.D. and Minoo B. Madon M V C A s s o c i a t i o n of C a l i f o r n i a MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA 660 J Street, Suite 480, Sacramento, CA 95814 Date of Publication - 2002 This is a publication of the MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA For other MVCAC publications or further informaiton, contact: MVCAC 660 J Street, Suite 480 Sacramento, CA 95814 Telephone: (916) 440-0826 Fax: (916) 442-4182 E-Mail: [email protected] Web Site: http://www.mvcac.org Copyright © MVCAC 2002. All rights reserved. ii Arthropods of Public Health Significance CONTENTS PREFACE ........................................................................................................................................ v DIRECTORY OF CONTRIBUTORS.............................................................................................. vii 1 EPIDEMIOLOGY OF VECTOR-BORNE DISEASES ..................................... Bruce F. Eldridge 1 2 FUNDAMENTALS OF ENTOMOLOGY.......................................................... Richard P. Meyer 11 3 COCKROACHES ........................................................................................... -

Role of Demodex Infestation in Blepharitis and Coconut Oil As a Treatment Option
Indian Journal of Clinical and Experimental Ophthalmology 2020;6(2):270–275 Content available at: iponlinejournal.com Indian Journal of Clinical and Experimental Ophthalmology Journal homepage: www.innovativepublication.com Original Research Article Role of demodex infestation in blepharitis and coconut oil as a treatment option Suresha A R1, Sadhwini M H1,* 1Dept. of Ophthalmology, JJM Medical College, Davangere, Karnataka, India ARTICLEINFO ABSTRACT Article history: Purpose: To assess incidence of demodex species, correlate ocular symptomatology, evaluate efficacy of Received 03-01-2020 coconut oil as treatment method in all types of blepharitis. Accepted 06-02-2020 Materials and Methods: 30 patients with anterior & mixed blepharitis, meibomian gland dysfunction Available online 16-06-2020 & non-specific irritation were enrolled for study. History taken & examined clinically. 2 lashes/lid were sampled & mounted on slides with normal saline & observed under light microscope. Number of mites counted. Patients positive for demodex were treated with coconut oil application over lid margins & Keywords: reviewed after 3 weeks. Anterior blepharitis Results: Incidence of demodex was 40% & it increased with age. Demodex was commonly associated with Demodex infestation meibomian gland dysfunction, non-specific irritation, madarosis, cloudy & toothpaste like meibum quality. Meibomian gland dysfunction Burning sensation and itching were common complaints. At 3rd week, all patients were symptom-free. Non-specific irritation Mite count dropped by 52.8% but were not eliminated. Conclusion: Demodex infestation is often overlooked but it is associated with about half of blepharitis cases. Hence further evaluation should be considered. Coconut oil is an easily available mode of treatment & helps reduce symptoms and mite counts. © 2020 Published by Innovative Publication. -

Parasitology JWST138-Fm JWST138-Gunn February 21, 2012 16:59 Printer Name: Yet to Come P1: OTA/XYZ P2: ABC
JWST138-fm JWST138-Gunn February 21, 2012 16:59 Printer Name: Yet to Come P1: OTA/XYZ P2: ABC Parasitology JWST138-fm JWST138-Gunn February 21, 2012 16:59 Printer Name: Yet to Come P1: OTA/XYZ P2: ABC Parasitology An Integrated Approach Alan Gunn Liverpool John Moores University, Liverpool, UK Sarah J. Pitt University of Brighton, UK Brighton and Sussex University Hospitals NHS Trust, Brighton, UK A John Wiley & Sons, Ltd., Publication JWST138-fm JWST138-Gunn February 21, 2012 16:59 Printer Name: Yet to Come P1: OTA/XYZ P2: ABC This edition first published 2012 © 2012 by by John Wiley & Sons, Ltd Wiley-Blackwell is an imprint of John Wiley & Sons, formed by the merger of Wiley’s global Scientific, Technical and Medical business with Blackwell Publishing. Registered Office John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030-5774, USA For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell. The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. -

Acne Vulgaris, Rosacea, Seborrheic Dermatitisଝ,ଝଝ
An Bras Dermatol. 2020;95(2):187---193 Anais Brasileiros de Dermatologia www.anaisdedermatologia.org.br INVESTIGATION Demodex folliculorum infestations in common facial dermatoses: acne vulgaris, rosacea, seborrheic dermatitisଝ,ଝଝ ∗ Ezgi Aktas¸ Karabay , Aslı Aksu C¸erman Department of Dermatology and Venereology, Faculty of Medicine, Bahc¸es¸ehir University, Istanbul, Turkey Received 18 March 2019; accepted 26 August 2019 Available online 12 February 2020 Abstract KEYWORDS Background: Demodex mites are found on the skin of many healthy individuals. Demodex mites Acne vulgaris; in high densities are considered to play a pathogenic role. Dermatitis, Objective: To investigate the association between Demodex infestation and the three most seborrheic; common facial dermatoses: acne vulgaris, rosacea and seborrheic dermatitis. Rosacea Methods: This prospective, observational case-control study included 127 patients (43 with acne vulgaris, 43 with rosacea and 41 with seborrheic dermatitis) and 77 healthy controls. The presence of demodicosis was evaluated by standardized skin surface biopsy in both the patient and control groups. Results: In terms of gender and age, no significant difference was found between the patients and controls (p > 0.05). Demodex infestation rates were significantly higher in patients than in controls (p = 0.001). Demodex infestation rates were significantly higher in the rosacea group than acne vulgaris and seborrheic dermatitis groups and controls (p = 0.001; p = 0.024; p = 0.001, respectively). Demodex infestation was found to be significantly higher in the acne vulgaris and seborrheic dermatitis groups than in controls (p = 0.001 and p = 0.001, respectively). No difference was observed between the acne vulgaris and seborrheic dermatitis groups in terms of demodicosis (p = 0.294). -

Complete Mitochondrial Genomes of the Human Follicle Mites Demodex Brevis and D
Palopoli et al. BMC Genomics 2014, 15:1124 http://www.biomedcentral.com/1471-2164/15/1124 RESEARCH ARTICLE Open Access Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species Michael F Palopoli*, Samuel Minot, Dorothy Pei, Alicia Satterly and Julie Endrizzi Abstract Background: Follicle mites of the genus Demodex are found on a wide diversity of mammals, including humans; surprisingly little is known, however, about the evolution of this association. Additional sequence information promises to facilitate studies of Demodex variation within and between host species. Here we report the complete mitochondrial genome sequences of two species of Demodex known to live on humans—Demodex brevis and D. folliculorum—which are the first such genomes available for any member of the genus. We analyzed these sequences to gain insight into the evolution of mitochondrial genomes within the Acariformes. We also used relaxed molecular clock analyses, based on alignments of mitochondrial proteins, to estimate the time of divergence between these two species. Results: Both Demodex genomes shared a novel gene order that differs substantially from the ancestral chelicerate pattern, with transfer RNA (tRNA) genes apparently having moved much more often than other genes. Mitochondrial tRNA genes of both species were unusually short, with most of them unable to encode tRNAs that could fold into the canonical cloverleaf structure; indeed, several examples lacked both D- and T-arms. Finally, the high level of sequence divergence observed between these species suggests that these two lineages last shared a common ancestor no more recently than about 87 mya. -
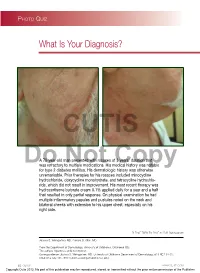
Demodex Folliculitis
Photo Quiz What Is Your Diagnosis? CUTIS A 79-year-old man presented with rosacea of 3 years’ duration that Dowas refractory toNot multiple medications. His medicalCopy history was notable for type 2 diabetes mellitus. His dermatologic history was otherwise unremarkable. Prior therapies for his rosacea included minocycline hydrochloride, doxycycline monohydrate, and tetracycline hydrochlo- ride, which did not result in improvement. His most recent therapy was hydrocortisone butyrate cream 0.1% applied daily for a year and a half that resulted in only partial response. On physical examination he had multiple inflammatory papules and pustules noted on the neck and bilateral cheeks with extension to his upper chest, especially on his right side. PLEASE TURN TO PAGE 65 FOR DISCUSSION Joshua S. Weingartner, MD; Pamela S. Allen, MD From the Department of Dermatology, University of Oklahoma, Oklahoma City. The authors report no conflict of interest. Correspondence: Joshua S. Weingartner, MD, University of Oklahoma Department of Dermatology, 619 NE 13th St, Oklahoma City, OK 73104 ([email protected]). 62 CUTIS® WWW.CUTIS.COM Copyright Cutis 2012. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Photo Quiz Discussion The Diagnosis: Demodex Folliculitis CUTIS he most common ectoparasites in humans an increased number of sebaceous glands, such as the are Demodex mites.1 The mite Demodex face, scalp, neck, eyelids, and upper chest.7 In most Tfolliculorum was first discovered in cerumen cases, the presence of these mites is asymptomatic in 1841 by the anatomist Jakob Henle; the mite was and causes no clinical findings. -

Anti‑Demodectic Effects of Okra Eyelid Patch in Demodex Blepharitis Compared with Tea Tree Oil
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 338, 2021 Anti‑demodectic effects of okra eyelid patch in Demodex blepharitis compared with tea tree oil WENTING LIU1,2 and LAN GONG1,2 1Department of Ophthalmology and Vision Science, The Eye, Ear, Nose and Throat Hospital of Fudan University; 2NHC Key Laboratory of Myopia, Laboratory of Myopia, Chinese Academy of Medical Sciences, Fudan University, Shanghai 200031, P.R. China Received January 7, 2020; Accepted January 18, 2021 DOI: 10.3892/etm.2021.9769 Abstract. Demodex infection gradually develops to Demodex rate of complete Demodex eradication in the treatment group blepharitis, which is characterized as chronic inflammation of (11/27, 40.74%) was slightly lower than that in the control the eyelid and meibomian gland (MG) and ultimately leads group (12/25, 48%), but there was no significant difference to MG dysfunction. In the present prospective study, the between the two groups (χ2=0.277, P=0.598). Regarding anti‑demodectic effects of an okra eyelid patch in patients the other ocular parameters, no significant difference was Demodex blepharitis were investigated. A total of 52 patients observed in the TBUT, meibum quality and MGE between the with Demodex blepharitis with ocular discomfort were two groups (P<0.05). TTO group has a significantly improve‑ recruited. Patients were randomized to receive either an okra ment compared with Okra group in terms of SIT (P=0.035) eyelid patch treatment (treatment group, n=27) or tea tree and CFS (P=0.023). In conclusion, okra eyelid patch treat‑ oil (TTO) eye care patch treatment (control group, n=25) for ment is able to significantly eradicate ocular Demodex as three months. -

Emtree Updates
Emtree Terms Changed in May 2019 1 Emtree Terms Added and Changed (May 2019) This is an overview of new terms added and changes made in the second Emtree release in 2019. Overall, Emtree has grown by 526 preferred terms (155 drug terms and 371 non-drug terms) compared with the previous version released in January 2019. In total Emtree now counts 82970 preferred terms. Because the terms added include replacements for existing preferred terms (which become synonyms of the new terms) as well as completely new concepts, the number of terms added exceeds the net growth in Emtree. Other changes could include the merging of two or more existing preferred terms into a single concept. The terms added and changed are summarized below and specified in detail on the following pages. Emtree Terms Added in May 2019 567 new terms (including 41 replacement terms and promoted synonyms) have been added to Emtree as preferred terms in version May 2019 (compared to January 2019): ◼ 176 drug terms (terms assigned to the Chemicals and Drugs facet). ◼ 391 non-drug terms (terms not assigned as Chemicals and Drugs). The new terms (including the replacement terms and the promoted synonyms) are listed as Terms Added on the following pages. Note that many of these terms will have been indexed prior to 2019 (typically as candidate terms), sometimes for several years, before they were added to Emtree. Emtree Terms Changed in May 2019 41 terms (21 drug terms and 20 non-drug terms) from Emtree January 2019 have been replaced by 39 different terms in May 2019 (21 drug terms and 18 non-drug terms).