Litigation Relevant to Regulation of Novel and Emerging Nicotine and Tobacco Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Electronic Cigarette & Vaporizer Market
EUROPEAN ELECTRONIC CIGARETTE & VAPORIZER MARKET Size, Share, Analysis & Forecast: 2015-2025 European Electronic Cigarette & E Vapor Market Size, Share, Analysis & Forecast: 2015-2025 BIS Research is a leading market intelligence and technology research company. BIS Research publishes in-depth market intelligence reports focusing on the market estimations, technology analysis, emerging high-growth applications, deeply segmented granular country-level market data and other important market parameters useful in the strategic decision making for senior management. BIS Research provides multi-client reports, company profiles, databases, and custom research services. Copyright © 2015 BIS Research All Rights Reserved. This document contains highly confidential information and is the sole property of BIS Research. Disclosing, copying, circulating, quoting or otherwise reproducing any or all contents of this document is strictly prohibited. Access to this information is provided exclusively for the benefit of the people or organization concerned. It may not be accessed by, or offered whether for sale or otherwise to any third party. BIS Research Sample Pages 2 European Electronic Cigarette & E Vapor Market Size, Share, Analysis & Forecast: 2015-2025 1 E-CIGARETTE MARKET DYNAMICS 1.1 INTRODUCTION The market dynamics section of the report examines the diverse factors which govern the production and consumption of e-cigarettes in the European market. This analysis will provide an in-depth understanding of the direction in which the market is headed and the impacts of various factors on it. This section covers the market dynamics – namely the drivers, challenges, and the opportunities in the electronic cigarette market, listing and analyzing several factors that positively and negatively affect it. -

Harm Reduction Journal Biomed Central
Harm Reduction Journal BioMed Central Research Open Access My first time: initiation into injecting drug use in Manipur and Nagaland, north-east India Michelle Kermode*1, Verity Longleng2, Bangkim Chingsubam Singh2, Jane Hocking3, Biangtung Langkham2 and Nick Crofts1 Address: 1Nossal Institute for Global Health, University of Melbourne, Carlton, Victoria, Australia, 2c/- Project ORCHID, CBCNEI Mission Compound, Guwahati, Assam, India and 3Key Centre for Women's Health, University of Melbourne, Carlton, Victoria, Australia Email: Michelle Kermode* - [email protected]; Verity Longleng - [email protected]; Bangkim Chingsubam Singh - [email protected]; Jane Hocking - [email protected]; Biangtung Langkham - [email protected]; Nick Crofts - [email protected] * Corresponding author Published: 5 December 2007 Received: 3 July 2007 Accepted: 5 December 2007 Harm Reduction Journal 2007, 4:19 doi:10.1186/1477-7517-4-19 This article is available from: http://www.harmreductionjournal.com/content/4/1/19 © 2007 Kermode et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: The north-east Indian states of Manipur and Nagaland are two of the six high HIV prevalence states in the country, and the main route of HIV transmission is injecting drug use. Understanding the pathways to injecting drug use can facilitate early intervention with HIV prevention programs. While several studies of initiation into injecting drug use have been conducted in developed countries, little is known about the situation in developing country settings. -
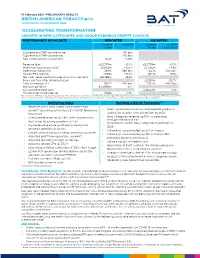
FY 2020 Announcement.Pdf
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -

Spring 2003 Vol
ISSN 1536-4003 University of California, Berkeley Center forSlavic and East European Studies Newsletter Notes from the Director Spring 2003 Vol. 20, No. 1 In these unsettled times, it behooves us more than ever to understand the In this issue: causes and consequences of violence inflicted by extremist groups. The Notes from the Director .....................1 United States is not alone in facing profound challenges following the Bear Treks ........................................2 terrorist attacks of September 11, 2001. Like us, our West European allies are Berkeley-Stanford Conference ...........3 struggling to find a reasonable balance between respect for civil liberties Spring Courses .................................4 and freedom of expression on the one hand and the need to protect society Michael Carpenter from acts of terrorism carried out in the name of extremist ideologies. So, too, The Modern Slave Trade in is Russia wrestling with these issues, as highlighted most dramatically by Post-Communist Europe ...................5 the ongoing violence and human rights abuses being perpetrated by federal Michael Kunichika forces in Chechnya, as well as human rights violations and acts of terrorism A Window On the Lake: On and by the Chechen resistance, such as the recent hostage-taking incident in Around Stalin's Belomorkanal ...........9 Moscow. Nor should it be assumed that the only extremist challenge in Campus Visitors ............................. 16 Russia, or indeed elsewhere, comes from Islamist extremists. As Vladimir Christine Kulke Putin put it in his state of the nation address on April 18, 2002: “The growth Book Review: Historical Atlas of of extremism presents a serious threat to stability and public safety in our Central Europe ............................... -

Immediate Effect of Tobacco Chewing in the Form of 'Paan'
April-June 1988 Ind. J. Physiol. Pharmac. e and high altitude stress on humans. behaviour and pituitary adrenal axis IMMEDIATE EFFECT OF TOBACCO CHEWING IN THE FORM of corticosteroids in lactating goats OF 'PAAN' ON CERTAIN CARDIO-RESPIRATORY PARAMETERS r lactating rats : Parallel changes P. K. NANDA AND M. M. SHARMA I-hydroxy corticosteroids in human Department of Physiology, Indira Gandhi Medical College, Shimla - 171 001 on serum transaminase and lactic of Physiological Sciences, New Delhi (Received on May 17, 1987 ) ofstress on serum enzyme levels in Summary: Immediate effect of tobacco in the form of chewing was evaluated in 40 healthy males (mean age 26.27 yrs.) not habituated to tobacco, who were given paan containing 200 mg of of drug metabolizing enzymes and tobacco to chew (group T). Heart rate (HR), blood pressure (BP), forced vital capacity (FVC), nal stress, Ind. J. Med, Res., 83 : FEV} and peak expiratory flow rate (PEFR) were measured twice for each subject, once before chewing and again immediately after completion of chewing. Another 24 age and sex matched ry and clearace in sheep before and controls (group C) were given paan without tobacco to chew and cardiorespiratory parameters 1970. were recorded as for group T subjects. Electrocardiography was recorded in lO group T and 10 ase Process" Ed. J. M. Raamsey , group C subjects. Effect of tobacco chewing was also evaluated in 10 habitual tobacco chewers. Results showed statistically significant increments in HR and BP as well as a decline in T wave of serum Glutamic oxalacetic and amplitude in ECG following tobacco chewing (group T subjects). -

IMS Data Reference Tables
IMS REFERENCE DATA – VERSION 1.7 TABLES 1. Substance .............................................................................................................................................................................. 2 2. Local Authority ....................................................................................................................................................................... 6 3. Drug Action Team (DAT) ...................................................................................................................................................... 13 4. Nationality ........................................................................................................................................................................... 17 5. Ethnicity ............................................................................................................................................................................... 22 6. Sexual Orientation ............................................................................................................................................................... 22 7. Religion or Belief .................................................................................................................................................................. 22 8. Employment Status .............................................................................................................................................................. 23 9. Accommodation Status -

OCTOBER 2019 Network Bulletin an Important Message from Unitedhealthcare to Health Care Professionals and Facilities
OCTOBER 2019 network bulletin An important message from UnitedHealthcare to health care professionals and facilities. Enter UnitedHealthcare respects the expertise of the physicians, health care professionals and their staff who participate in our network. Our goal is to support you and your patients in making the most informed decisions regarding the choice of quality and cost-effective care, and to support practice staff with a simple and predictable administrative experience. The Network Bulletin was developed to share important updates regarding UnitedHealthcare procedure and policy changes, as well as other useful administrative and clinical information. Where information in this bulletin conflicts with applicable state and/or federal law, UnitedHealthcare follows such applicable federal and/or state law. UnitedHealthcare Network Bulletin October 2019 Table of Contents Front & Center PAGE 3 Stay up to date with the latest news and information. UnitedHealthcare Commercial PAGE 22 Learn about program revisions and requirement updates. UnitedHealthcare Community Plan PAGE 29 Learn about Medicaid coverage changes and updates. UnitedHealthcare Medicare Advantage PAGE 35 Learn about Medicare policy, reimbursement and guideline changes. UnitedHealthcare Affiliates PAGE 37 Learn about updates with our company partners. PREV NEXT 2 | For more information, call 877-842-3210 or visit UHCprovider.com. UnitedHealthcare Network Bulletin October 2019 Table of Contents Front & Center Stay up to date with the latest news and information. Smart Edits Help Speed Up Radiology Program Outpatient Injectable Your Claims Cycle Procedure Code Changes Chemotherapy and Our Smart Edits claims tool catches Effective Jan. 1, 2020, Related Cancer Therapies errors and gives you an opportunity UnitedHealthcare will update Prior Authorization/ to resolve and resubmit a claim the procedure code list for the Notification Updates before it enters the claims cycle. -

Tobacco Use: a Smart Guide
1 Tobacco Use: A Smart Guide “STOPPING TAKES HARD WORK AND A LOT OF EFFORT, BUT – YOU CAN STOP TOBACCO” THE PROCESS OF QUITTING Do you find quitting tobacco difficult? The reason you continue to use tobacco is not because you are weak-willed or irresponsible, but because you are addicted. Nicotine is said to be more addictive than brown sugar (heroin) or cocaine. That is why people find it so difficult to stop, once they are habituated. The quitting process involves three steps: A. Preparation before quitting B. Actual quitting and C. Life after quitting. This manual will guide you through each of these steps. YOU NEED TO KNOW Before trying to quit tobacco you need to know a few facts about tobacco: • Tobacco comes in different forms….and all contain nicotine, the addictive substance. • Smoking forms of tobacco are beedis, cigarettes, cigars, chuttas, dhumti, pipe, hooklis, and hookah. • Smokeless forms of tobacco include chewing paan (betel quid) with zarda (tobacco), guthka, pan masala, manipuri tobacco, mawa, khaini, kaddi pudi, chewing tobacco leaves, mishri, gul, snuff, tobacco tooth paste and as tobacco water. Tobacco Facts • Tobacco is the leading cause of preventable death. • Each year tobacco kills 40,00,000 people. 2500 Indians die EVERYDAY due to tobacco related diseases. Deaths from tobacco use world wide are more than that from cocaine, heroin, alcohol, fires, accidents, murder, suicide, and AIDS COMBINED. • If you use tobacco, you are likely to die 15 years earlier. Tobacco Use: A Smart Guide 2 • Tobacco affects all the organs in the body from head to toe. -

Opioid Prescriber Reference Guide
Community Plan of Pennsylvania (Medicaid) Quick reference guide Opioid overutilization prevention and opioid use disorder treatment programs for UnitedHealthcare Community Plan of Pennsylvania (Medicaid) In response to the U.S. opioid epidemic, we’ve developed programs to help our members receive the care and treatment they need in safe and effective ways. We’ve based our measures on the Centers for Disease Control and Prevention’s (CDC) opioid treatment guidelines to help prevent overuse of short-acting and long-acting opioid medications. Please use this quick reference guide to learn more about what we offer. Concurrent Drug Utilization Review (cDUR) programs The cDUR program uses the pharmacy claims processing system to screen all prescriptions at the point of service and checks for possible inappropriate drug prescribing and utilization, as well as potentially dangerous medical implications or drug interactions. The program includes communication to the dispensing pharmacy at point-of-service through claims edits and messaging to the dispensing pharmacy at point of service. The pharmacist will need to address the clinical situation at the point of sale before entering appropriate National Council for Prescription Drug Programs (NCPDP) codes to receive an approved claim, unless otherwise stated below. • Combination opioids plus acetaminophen (APAP) limit THERDOSE Acetaminophen • Prevents doses of APAP greater than 4 grams per day Duplicate Therapy – • Alerts to concurrent use of multiple SAOs Short-Acting Opioids (SAOs) Duplicate Therapy -

Does Areca Nut Use Lead to Dependence? Vivek Benegal ∗, Ravi P
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Available online at www.sciencedirect.com Drug and Alcohol Dependence 97 (2008) 114–121 Does areca nut use lead to dependence? Vivek Benegal ∗, Ravi P. Rajkumar, Kesavan Muralidharan Deaddiction Centre, Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore 560029, India Received 14 February 2007; received in revised form 24 March 2008; accepted 24 March 2008 Available online 19 May 2008 Abstract Background: The areca nut is consumed by approximately 10% of the world’s population, and its consumption is associated with long-term health risks, with or without tobacco additives. However, it is not known whether its use is associated with a dependence syndrome, as is seen with other psychoactive substances. Objective: To examine whether areca nut usage (with or without tobacco additives) could lead to the development of a dependence syndrome. Methods: Three groups: [a] persons using areca nut preparations without tobacco additives [n = 98]; [b] persons using areca nut preparations with tobacco additives [n = 44]; and [c] ‘Non-users’ were systematically assessed using a checklist for the use of areca or areca + tobacco products, patterns of use, presence of a dependence syndrome in users, features of stimulant withdrawal and desired/beneficial effects. -

Decision of the Minister of Health No. 1853515 on Approval of The
Unofficial Translation 22 Um Al- Friday 22 Rabea al-Awwal 1440 A.H - 30 Decisions & Laws Um Al-Qura Qura November 2018 Year 96 Year 96, Vol. 4755 [logo] Ministry of Health Decisions of the Ministry of Health Decision of the Minister of Health No. 1853515 on 29/12/ 1439 AH Approval on the Amendment of the Executive Regulation of Tobacco Control Law The Minister of Health, According to the authorities vested in him; After reviewing the Tobacco Law issued by the Royal Decree No. (M/56) on 28/07/1436 A.H; After reviewing Article 19 of Clause 1 of the Executive Regulation of Tobacco Control Law, which set forth that "The Ministry of Health reviews the Regulation one year from its enforcement and amends the same as it may requires". And as dictated by the public interest. Decided that: First: Approve the amendment to the Executive Regulation of Tobacco Control Law according to the form attached herewith: Second: This Regulation shall be published in the Official Gazette and on the Minister website and shall be put into force as of the date of its publication. Respectfully, Minister of Health Tawfiq bin Fawzran Al Rabiah Unofficial Translation 22 Um Al- Friday 22 Rabea al-Awwal 1440 A.H - 30 Decisions & Laws Um Al-Qura Qura November 2018 Year 96 Year 96, Vol. 4755 Executive Regulation of Tobacco Control Law Article 1: Law: This Law aims to control tobacco, by applying all the necessary procedures and steps in the State, society and individuals; reducing all forms of smoking habits at different ages. -

Autumn 2007 Full Issue the .SU
Naval War College Review Volume 60 Article 1 Number 4 Autumn 2007 Autumn 2007 Full Issue The .SU . Naval War College Follow this and additional works at: https://digital-commons.usnwc.edu/nwc-review Recommended Citation Naval War College, The .SU . (2007) "Autumn 2007 Full Issue," Naval War College Review: Vol. 60 : No. 4 , Article 1. Available at: https://digital-commons.usnwc.edu/nwc-review/vol60/iss4/1 This Full Issue is brought to you for free and open access by the Journals at U.S. Naval War College Digital Commons. It has been accepted for inclusion in Naval War College Review by an authorized editor of U.S. Naval War College Digital Commons. For more information, please contact [email protected]. 1 Autumn 2007 60, Number 4 Volume Naval War College: Autumn 2007 Full Issue NAVAL WAR COLLEGE REVIEW Published by U.S. Naval War College Digital Commons, 2007 NAVAL WAR COLLEGE REVIEW Autumn 2007 R COL WA LEG L E A A I V R A N O T C I V I R A M S U S E B I T A T R T I H S E V D U E N T I Color profile: Disabled Composite Default screen Naval War College Review, Vol. 60 [2007], No. 4, Art. 1 Cover The Kongo-class guided-missile destroyer JDS Chokai (DDF 176) of the Japan Mar- itime Self-Defense Force alongside USS Kitty Hawk (CV 63) on 10 December 2002. The scene is evocative of one of the many levels at which the “thousand-ship navy,” examined in detail in this issue by Ronald E.