Introductory Guide Meddra Version 21.0
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Science-5-Nov-16-20 Circulatory-System.Pdf
Grade 5 Science Week of November 16 – November 20 Circulatory System Most of the cells inside of your body do not move. If a cell is hungry or needs to get rid of waste, it can’t simply move itself to the part of your body where it needs to go. Instead, your body must bring the food to your cells and take the waste away from them. By using billions of tiny tubes the circulatory system transports substances around our bodies. It delivers essential nutrients to every cell, and it transports waste products to waste-disposal sites—the lungs, the skin, and the kidneys. The circulatory system is an organ system that includes the heart, the blood vessels, and the blood itself. It has three functions: 1. to transport materials (i.e., nutrients and oxygen) and cells from one place to another 2. to defend the body against invasion by harmful organisms by taking white blood cells to an area of injury or infection 3. to maintain a constant body temperature Introduction Your body has a network of blood vessels—hollow tubes—that move blood and nutrients. A pumping organ—the heart—pushes blood through this network of vessels. Watch this video to start looking at this incredible system: https://youtu.be/tF9-jLZNM10 Complete the following. 1) Fill in the blanks: 1. Most of the cells inside of your body ________________ . If a cell is _____________ or needs to get rid of ______________, it can’t simply move itself to the part of your body where it needs to go. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -
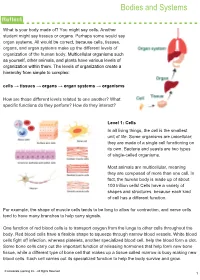
Bodies and Systems
Bodies and Systems What is your body made of? You might say cells. Another student might say tissues or organs. Perhaps some would say organ systems. All would be correct, because cells, tissues, organs, and organ systems make up the different levels of organization of the human body. Multicellular organisms such as yourself, other animals, and plants have various levels of organization within them. The levels of organization create a hierarchy from simple to complex: cells → tissues → organs → organ systems → organisms How are those different levels related to one another? What specific functions do they perform? How do they interact? Level 1: Cells In all living things, the cell is the smallest unit of life. Some organisms are unicellular; they are made of a single cell functioning on its own. Bacteria and yeasts are two types of single-celled organisms. Most animals are multicellular, meaning they are composed of more than one cell. In fact, the human body is made up of about 100 trillion cells! Cells have a variety of shapes and structures, because each kind of cell has a different function. For example, the shape of muscle cells tends to be long to allow for contraction, and nerve cells tend to have many branches to help carry signals. One function of red blood cells is to transport oxygen from the lungs to other cells throughout the body. Red blood cells have a flexible shape to squeeze through narrow blood vessels. White blood cells fight off infection, whereas platelets, another specialized blood cell, help the blood form a clot. -

The Respiratory System Pharyngeal Tonsil the Respiratory System Is Associated with Breathing
EMT-I_Ch05_114-239 9/25/04 1:34 PM Page 201 Chapter 5 The Human Body 201 The Respiratory System Pharyngeal tonsil The respiratory system is associated with breathing, Palatoglossal gas exchange, and the entrance of air into the body. The arch organs and structures in the respiratory system are Uvula divided into the upper and lower airways. The upper airway includes the mouth, nasal cavity, and oral cav- Palatine tonsil ity, and the lower airway includes the larynx, trachea, Palatopharyngeal bronchi, bronchioles, and alveoli. arch Lingual tonsil The Upper Airway Figure 5-111 The tonsils. A human can breathe air into the body through either the nose, also called the nasal cavity or nasopharynx, or through the mouth, also called the oral cavity or quite large in the infant and decreases in size with oropharynx. The nasopharynx and oropharynx connect age. Often, there is no discernible thymic tissue in posteriorly to form a cavity called the pharynx older adults. The thymus produces lymphocytes, which Figure 5-112 ᮢ . move to other lymph tissues to help fight infection. The nasopharynx extends from the internal nares to The thymus plays a major role in immunity, especially the uvula (a small fleshy mass that hangs from the soft in early life. palate). The oropharynx extends from the uvula to the A Artery Vein Alveolus Nasal cavity Bronchiole Mouth (oral cavity) Pharynx Larynx Capillary network Trachea Left bronchus Right bronchus B Alveoli Terminal bronchiole Capillaries Figure 5-112 The respiratory system. A. The upper and lower airway divisions of the respiratory system. The insert shows a higher magnification of the alveoli, where oxygen and carbon dioxide exchange occurs. -

From Cells to Tissue to Organs to Organ Systems There Are Many Kinds of Cells in the Human Body, Such As Muscle Cells, Bone Cells, Skin Cells, Blood Cells, Etc
From Cells to Tissue to Organs to Organ Systems There are many kinds of cells in the human body, such as muscle cells, bone cells, skin cells, blood cells, etc. Each kind of cell acts a little differently to suit its role in the body. For instance, muscles cells can stretch and snap back into shape (recoil) to suit the way a muscle needs function A nerve cell, on the other hand, doesn’t stretch or extend, but already has a very, very long tail (axon) down which a signal can be sent from one part of the body to another. The smallest units of the body – the cells – are individually too small to be seen by the naked eye. Yet when many like cells are together, they form a tissue. Tissues are groups of cells with a common structure and function. There are four main tissues in the body – epithelium, muscle, connective tissue and nervous tissue. Epithelium (or epithelial tissue) is found all over the body with several functions. In the skin it protects us from the outside world, in the stomach and intenstines it absorbs. In the kidney it filters and in the glands it secretes. Muscle tissue is responsible for body movement, moves blood, food, waste through body’s organs, and is responsible for mechanical digestion. Connective tissue wraps around, supports, cushions and protects organs. It stores nutrients and gives the skin strength. As tendons and ligaments, it protects joints and attaches muscles to bone and each other. It includes some specialized tissues including cartiage, bone and blood. -

The Study of Human Anatomy
The Study of Human Anatomy Chapter 1 Chapter Outline An overview of Anatomy: a) Define anatomy and physiology b) learn subdivisions of anatomy c) methods of study d) organ systems e) levels of structural organization Gross Anatomy: anatomical position; anatomical terminology, body cavities, 9 regions and 4 quadrants of abdomen, Microscopic Anatomy: Light and Electron microscopy Clinical Anatomy: an introduction to medical imaging techniques An Overview of Anatomy Anatomy is the study of Structure of body and body parts and their relationships to one another. (anatome = dissection). Morphology Morphe = form, logos = study Physiology is the study of Function of body and body parts. Structure supports function. For example, the cornea of eye is transparent and curved. It allows light to pass through and focuses it as image. Anatomy Major subdivisions of anatomy Gross Anatomy is the study of body surface, regions, and sections. It studies organs and their relationship to one another. Microscopic Anatomy studies the cells and tissues. Cytology is the study of cells and Histology is the study of tissues. Systemic and Regional Anatomy Methods of study Inspection is to look keenly on body surface or parts. Nails, eyes, tongue, injury marks Palpation means feeling a structure with hands. Pulse Auscultation means listening to natural sound made by the body. Heart or lung sounds with stethoscope. Dissection is cutting open the body and internal organs Exploratory Surgery of past is now replaced by medical imaging techniques – Radiography (X-rays), CT scan, Angiography, Sonography (Ultrasound scanning), and MRI X-rays are invasive, can cause mutations; Sonography and MRI are noninvasive. -

1997 Documentation Guidelines for Evaluation and Management Services
1997 DOCUMENTATION GUIDELINES FOR EVALUATION AND MANAGEMENT SERVICES TABLE OF CONTENTS Introduction ....................................................................................................…… 2 What Is Documentation and Why Is it Important?............................………. 2 What Do Payers Want and Why? .......................................................……… 2 General Principles of Medical Record Documentation ..................................... 3 Documentation of E/M Services........................................................................... 4 Documentation of History .................................................................................... 5 Chief Complaint (CC) ..................................................................................... 6 History of Present Illness (HPI) ..................................................................... 7 Review of Systems (ROS) .............................................................................. 8 Past, Family and/or Social History (PFSH) ................................................... 9 Documentation of Examination ........................................................................... 10 General Multi-System Examinations ............................................................ 11 Single Organ System Examinations ............................................................ 12 Content and Documentation Requirements ................................................ 13 General Multi-System Examination ……….............................................. -

Cell Organization All Living Things Are Made of One Or More Cells
Cell Organization All living things are made of one or more cells. Cells are the basic building blocks of all organisms. The organization of cells into complex structures allows for the wide variety of life found in multicellular organisms. Organization in Multicellular Organisms From simplest to most complex, the proper levels of organization in multicellular organisms are: The levels of biological organization in order from smallest to largest are: atom → molecule → macromolecule → organelle → cell → tissue → organ → organ system → whole organism. Organelles are specialized subunits in the cell, which each have their own specific function. They are usually enclosed in their own lipid membrane. There are many types of organelles, such as ribosomes, nuclei, endoplasmic reticulum, and lysosomes. Cells are the structural and functional units of all living organisms. Organisms can be made up of one cell, like bacteria, or many cells, like animals. Cells specialize depending upon which part of the body they are located. All cells come from other cells, and they divide by mitosis or meiosis. Cells contain organelles and the genetic information of an organism. Tissues are composed of many cells that work together to perform a specific function. Tissue covers most parts of an organism. There are several types of tissues, such as connective tissue, muscle tissue, nervous tissue, and epithelial tissue. Organs are composed of several tissues and perform one or more functions in the body. In most organs there is a unique 'main' type of tissue (such as the myocardium of the heart) and several other tissues that are found in many organs (such as connective tissue). -

Lab 9 - Vertebrate Organ Systems
Biology 18 Spring 2008 Lab 9 - Vertebrate Organ Systems Objectives: Understand the taxonomic relationships and major features of the chordates and the major classes of vertebrates Identify structures and their functions associated with major systems - nervous/sensory, respiratory, circulatory, digestive and excretory - in different vertebrates Learn the taxonomic relationships between groups, to understand when similarities between structures are based on history (homology) or convergence Introduction: As you will recall from the Introduction to Animal Diversity handout, organisms need to carry out many processes to survive and reproduce. During the next two weeks, you will examine the internal organs of vertebrates as a way of understanding their anatomy and physiology. As before, please approach your studies of vertebrate anatomy and physiology as suggested on pp. 1-2 of the Animal Diversity handout. Once again, you should understand that similarities or differences in vertebrate systems are often a reflection of the environment in which an animal lives and the type of food that it eats. Finally, think about how the anatomical and physiological solutions of vertebrates compare with those that you observed earlier in invertebrates. Textbook Reference Pages: pp. 717-718 and 722 -741 Phylum Chordata The Phylum Chordata contains the group most familiar to you – the vertebrates. The members of this phylum are distinguished by: 1) They are triploblastic, possessing an ectoderm and endoderm, and a middle tissue layer, the mesoderm. They are coelomate, possessing a true body cavity lined on all sides by mesoderm-derived tissues. 2) Chordates are bilaterally symmetrical. 3) They have a notochord, a flexible supportive rod that runs longitudinally through the dorsum. -

Internal Organs Including the Gonads (Testicles and Ovaries.) Day 5: Other Organ Systems Skeletal System
Medical Terminology Overview WEEK 1 https://www.youtube.co m/watch?v=0yjLJfz6saU 11 Human Organ Systems 1. Respiratory System 7. Lymphatic System 2. Digestive System 8. Endocrine System 3. Cardiovascular System 9. Nervous System 4. Urinary System 10.Reproductive System 5. Skeletal System 11.Exocrine System 6. Muscular System (Integumentary System) Day 1: Respiratory System Respiratory System The respiratory system includes air passages (airways) and the lungs. It is responsible for taking in oxygen and removing carbon dioxide. Lungs The lungs are a pair of spongy, air-filled organs located in the chest. Lungs take in oxygen from the atmosphere and moves it through the bloodstream. They also release carbon dioxide from the bloodstream back into the atmosphere. The right lung is bigger than the left lung—the left lung shares its space with the heart. Diaphragm The thoracic diaphragm is a sheet of skeletal muscle the separates the heart and lungs from the abdominal cavity. The diaphragm is the main muscle for breathing. As the diaphragm contracts, the lungs draw in air. Pharynx The technical name for your throat is pharynx. It is a tube that carries food to your esophagus and air to your windpipe and larynx. Larynx The larynx is the portion of the breathing, or respiratory, tract containing the vocal cords which produce vocal sound. It is located between the pharynx and the trachea. The larynx, also called the voice box, is a 2- inch-long, tube-shaped organ in the neck. We use the larynx when we breathe, talk, or swallow. Its outer wall of cartilage forms the area of the front of the neck referred to as the "Adams apple." The vocal cords, two bands of muscle, form a "V" inside the larynx. -

Anatomy Coloring Book
Chapter One: Introduction ANATOMICAL POSITION AND TERMS OF DIRECTION When studying the human body it is important to place the body in anatomical position. Anatomical a. _ a. _ position is described as the body facing you, feet placed together and flat on the floor. The head is held erect, arms straight by the side with palms facing forward. All references to the body are made as if the body is in this position so when you describe e. _ something as being above something ) else it is always with respect to the body being in anatomical position. The relative position of the parts of h.___ ( 1. _ the human body has specific terms. ....lII('---:-----':----;-~. Superior means above while ). k.-- inferior means below. Medial refers to being close to the midline while 1) lateral means to the side. Anterior I, \ or ventral is to the front while e _ posterior or dorsal is to the back. VI Superficial is near the surface while deep means to the core of the body. When working with the limbs, proximal means closer to the trunk while distal is to the ends of the extremities. Write the directional terms in the spaces provided and color in the arrows in reference to these terms. Note that these terms are somewhat different for four legged animals. f. __ b. __ g._----- b. __ ~V? )~ h.__ .. I 1 I. l«=~.. \! Answer Key: a. Superior, b. Inferior, c. Lateral, d. Medial, e. Proximal, f. Distal, g. Anatomical position, h Posterior, i. Anterior, j. Dorsal, k. -

ANATOMY of DIGESTIVE SYSTEM Ø Presented by
ANATOMY OF DIGESTIVE SYSTEM Ø Presented by : Raghad Mustafa Belgaseem Bobaker Malak Akraim 2 ILLOS:- 1.Define the digestive system 2.Describe the organ of digestive system 3.Mention the blood supply of each organ of digestive system 4.Explain accessory organ of digestive system 3 Ø Definition:- It is an organ system within humans which takes in food, digests it and absorb energy and nutrients, and expels the remaining waste as feces. 4 v Major organs of digestive system: 1) MOUTH 2) PHARYNX 3) ESOPHAGUS 4) STOMACH 5) SMALL INTESTINE 6) LARGE INTESTINE 5 Ø Accessory of digestive organs v Liver v Gallbladder v Pancreas v Salivary gland 6 Ø ANATOMY OF DIGESTIVE SYSTEM Ø MOUTH: it is the first part of alimentary canal that receive food and produce saliva 7 Ø TONGUE: Is a muscular organ covered by mucous membrane 8 Ø TEETH:- Ø Primary teeth Ø Permanent teeth 9 Ø THE PHARYNX The pharynx is the part of the throat that is behind the mouth and nasal cavity and above the esophagus and the larynx, or the tubes going down to the stomach and the lungs. 10 Ø pharynx is divided into 3 parts vNasopharynx v Oro pharynx vLaryngo pharynx 11 Ø THE ESOPHAGUS The esophagus is a muscular tube connecting the throat (pharynx) with the stomach. • Length: 25 cm • Diameter: 2 cm 12 Ø STOMACH • The stomach is a muscular organ located on the left side of the upper abdomen. 13 Ø The Small Intestine The Small Intestine Or Small Bowel is the Part of the Gastrointestinal Tracto Between The Stomach And The Large Intestine, Ø Length – 3m – 5m.