Lab 9 - Vertebrate Organ Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pond-Breeding Amphibian Guild
Supplemental Volume: Species of Conservation Concern SC SWAP 2015 Pond-breeding Amphibians Guild Primary Species: Flatwoods Salamander Ambystoma cingulatum Carolina Gopher Frog Rana capito capito Broad-Striped Dwarf Siren Pseudobranchus striatus striatus Tiger Salamander Ambystoma tigrinum Secondary Species: Upland Chorus Frog Pseudacris feriarum -Coastal Plain only Northern Cricket Frog Acris crepitans -Coastal Plain only Contributors (2005): Stephen Bennett and Kurt A. Buhlmann [SCDNR] Reviewed and Edited (2012): Stephen Bennett (SCDNR), Kurt A. Buhlmann (SREL), and Jeff Camper (Francis Marion University) DESCRIPTION Taxonomy and Basic Descriptions This guild contains 4 primary species: the flatwoods salamander, Carolina gopher frog, dwarf siren, and tiger salamander; and 2 secondary species: upland chorus frog and northern cricket frog. Primary species are high priority species that are directly tied to a unifying feature or habitat. Secondary species are priority species that may occur in, or be related to, the unifying feature at some time in their life. The flatwoods salamander—in particular, the frosted flatwoods salamander— and tiger salamander are members of the family Ambystomatidae, the mole salamanders. Both species are large; the tiger salamander is the largest terrestrial salamander in the eastern United States. The Photo by SC DNR flatwoods salamander can reach lengths of 9 to 12 cm (3.5 to 4.7 in.) as an adult. This species is dark, ranging from black to dark brown with silver-white reticulated markings (Conant and Collins 1991; Martof et al. 1980). The tiger salamander can reach lengths of 18 to 20 cm (7.1 to 7.9 in.) as an adult; maximum size is approximately 30 cm (11.8 in.). -

Ontogenetic Evidence for the Paleozoic Ancestry of Salamanders
EVOLUTION & DEVELOPMENT 5:3, 314–324 (2003) Ontogenetic evidence for the Paleozoic ancestry of salamanders Rainer R. Schocha and Robert L. Carrollb aStaatlilches Museum für Naturkunde, Rosenstein 1, D-70191 Stuttgart, Germany bRedpath Museum, McGill University, Montréal, Québec, Canada, H3A 2K6 Authors for correspondence (e-mail: [email protected], [email protected]) SUMMARY The phylogenetic positions of frogs, sala- tire developmental sequence from hatching to metamor- manders, and caecilians have been difficult to establish. phosis is revealed in an assemblage of over 600 Data matrices based primarily on Paleozoic taxa support a specimens from a single locality, all belonging to the genus monophyletic origin of all Lissamphibia but have resulted in Apateon. Apateon forms the most speciose genus of the widely divergent hypotheses of the nature of their common neotenic temnospondyl family Branchiosauridae. The se- ancestor. Analysis that concentrates on the character quence of ossification of individual bones and the changing states of the stem taxa of the extant orders, in contrast, configuration of the skull closely parallel those observed in suggests a polyphyletic origin from divergent Paleozoic the development of primitive living salamanders. These clades. Comparison of patterns of larval development in fossils provide a model of how derived features of the sala- Paleozoic and modern amphibians provides a means to mander skull may have evolved in the context of feeding test previous phylogenies based primarily on adult charac- specializations that appeared in early larval stages of mem- teristics. This proves to be highly informative in the case of bers of the Branchiosauridae. Larvae of Apateon share the origin of salamanders. -

Histological Observation of the External Gills of a Mexican Axolotl (Ambystoma Mexicanum) with Atypical Blood Vessels
Naturalistae 23: 47-52 (Feb. 2019) © 2019 by Okayama University of Science, PDF downloadable at http://www1.ous.ac.jp/garden/ Original paper Histological observation of the external gills of a Mexican axolotl (Ambystoma mexicanum) with atypical blood vessels Saki YOSHIDA1 and Kazuyuki MEKADA1* Abstract: The external gills of captive Mexican axolotl (Ambystoma mexicanum) sometimes develop atypical blood vessels, the cause of which is unknown. We observed the external gill filaments of an individual animal with dilated blood vessels that formed a semicircle within the filament tissue. The positioning of the swollen blood vessels compressed the adjacent capillaries and connective tissues. Normal external gill filaments in urodelans contain a blood-vessel system with afferent and efferent arterioles that connect to circumvent the outer gill periphery. We infer that the dilated blood vessels in the axolotl originated from these arterioles. I. Introduction et al. 2015, Nowoshilow et al. 2018, Page et al. 2013, Voss et al. 2015). Furthermore, the Mexican The Mexican axolotl (Ambystoma mexicanum) axolotl has gained widespread popularity as a pet is a tailed urodelan amphibian indigenous to (Lang 2013, Reiβ et al. 2015). Lake Xochimilco and Lake Chalco in Mexico Amphibian larvae have either external or (Zambrano et al. 2007). Over the past 50 years, it internal gills (Brunelli et al. 2009). Generally, has been used as a model organism in disciplines urodele larvae have external gills on both sides such as evolution, embryology, and regeneration of the neck until metamorphosis. However, the (Reiβ et al. 2015, Voss et al. 2009). Recently its axolotl does not metamorphose at sexual matu- genome has been sequenced to allow studies of rity, and instead retains its external gills (Bishop comparative genomics, quantitative trait locus 1994). -
Science-5-Nov-16-20 Circulatory-System.Pdf
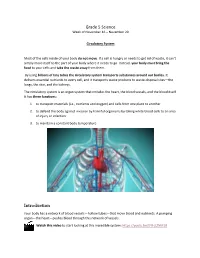
Grade 5 Science Week of November 16 – November 20 Circulatory System Most of the cells inside of your body do not move. If a cell is hungry or needs to get rid of waste, it can’t simply move itself to the part of your body where it needs to go. Instead, your body must bring the food to your cells and take the waste away from them. By using billions of tiny tubes the circulatory system transports substances around our bodies. It delivers essential nutrients to every cell, and it transports waste products to waste-disposal sites—the lungs, the skin, and the kidneys. The circulatory system is an organ system that includes the heart, the blood vessels, and the blood itself. It has three functions: 1. to transport materials (i.e., nutrients and oxygen) and cells from one place to another 2. to defend the body against invasion by harmful organisms by taking white blood cells to an area of injury or infection 3. to maintain a constant body temperature Introduction Your body has a network of blood vessels—hollow tubes—that move blood and nutrients. A pumping organ—the heart—pushes blood through this network of vessels. Watch this video to start looking at this incredible system: https://youtu.be/tF9-jLZNM10 Complete the following. 1) Fill in the blanks: 1. Most of the cells inside of your body ________________ . If a cell is _____________ or needs to get rid of ______________, it can’t simply move itself to the part of your body where it needs to go. -

Morphological Alterations in the External Gills of Some Tadpoles in Response to Ph
THIEME 142 Original Article Morphological Alterations in the External Gills of Some Tadpoles in Response to pH CaressaMaebhaThabah1 Longjam Merinda Devi1 Rupa Nylla Kynta Hooroo1 Sudip Dey2 1 Developmental Biology Laboratory, Department of Zoology, North- Address for correspondence Caressa Maebha Thabah, PhD in Eastern Hill University, Shillong, Meghalaya, India Zoology, Developmental Biology Laboratory, Department of Zoology, 2 Electron Microscope Laboratory, Sophisticated Analytical Instrument North-Eastern Hill University, Shillong 793022, Meghalaya, India Facility, North-Eastern Hill University, Shillong, Meghalaya, India (e-mail: [email protected]). J Morphol Sci 2018;35:142–152. Abstract Introduction Water pH affects the breeding, hatching, development, locomotion, mortality and habitat distributions of species in nature. The external gills of anuran tadpoles were studied by several authors in relation to abiotic factors. Exposure to low and high pH has been found to adversely affect the different tissues of various organisms. On that consideration, the present investigation was performed with tadpoles of the species Hyla annectans and Euphlyctis cyanophlyctis. Material and Methods The maximum and the minimum pH thresholds were determined prior to the detailed experiments on the effects of pH. The pH that demonstrated 50% mortality was taken as the minimum and maximum pH thresholds. The hatchlings of both the species were then subjected to different pH (based on the minimum and maximum pH thresholds). After 48 hours of exposure, the external gills of the hatchlings were anesthetized and observed under a scanning electron microscope. Keywords Results After 48 hours, clumping, overlapping and curling of the secondary filaments ► Hyla annectans of the external gills and epithelial lesions in response to both acidic and alkaline pH ► Euphlyctis were observed. -

External Gills and Adaptive Embryo Behavior Facilitate Synchronous Development and Hatching Plasticity Under Respiratory Constraint
3627 The Journal of Experimental Biology 211, 3627-3635 Published by The Company of Biologists 2008 doi:10.1242/jeb.020958 External gills and adaptive embryo behavior facilitate synchronous development and hatching plasticity under respiratory constraint Jessica R. Rogge and Karen M. Warkentin* Department of Biology, Boston University, Boston, MA 02215, USA 'Author for correspondence (e-mail: [email protected]) Accepted 22 September 2008 SUMMARY Plasticity in hatching timing allows embryos to balance egg- and larval-stage risks, and depends on the ability of hatching- competent embryos to continue developing in the egg. Hypoxia can slow development, kill embryos and induce premature hatching. For terrestrial eggs of red-eyed treefrogs, the embryonic period can extend ~50% longer than development to hatching competence, and development is synchronous across perivitelline oxygen levels (Po2) ranging from 0.5-16.5 kPa. Embryos maintain large external gills until hatching, then gills regress rapidly. We assessed the respiratory value of external gills using gill manipulations and closed-system respirometry. Embryos without external gills were oxygen limited in air and hatched at an external Po2 of 17kPa, whereas embryos with gills regulated their metabolism and remained in the egg at substantially lower Po2. By contrast, tadpoles gained no respiratory benefit from external gills. We videotaped behavior and manipulated embryos to test if they position gills near the air-exposed portion of the egg surface, where PQ2 is highest. Active embryos remained stationary for minutes in gills-at-surface positions. After manipulations and spontaneous movements that positioned gills in the 02-poor region of the egg, however, they returned their gills to the air-exposed surface within seconds. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

Vertebrate Respiratory System Gills
Dr. Medhavi Sudarshan Assist Prof nd B.SC 2 Year, Paper IV Deapt of Zoology JNL College , Khagaul Comparative Anatomy – Vertebrate Respiratory System Respiratory system is a system consisting of specific organs and structures used for the process of respiration in an organism. Respiration is the process of obtaining oxygen from the external environment & eliminating CO2. External respiration - oxygen and carbon dioxide exchanged between the external environment & the body cells Internal respiration - cells use oxygen for ATP production (& produce carbon dioxide in the process) In vertebrates the skin may be respiratory (e.g., anurans), while in some fishes and aquatic turtles, the vascular rectum or cloaca is respiratory. But there are two main types of respiratory organs- gills for aquatic respiration and lungs for aerial respiration. Both gills and lungs may occur in the same animal. Accessory respiratory organs are also present in some vertebrates. In both kinds of respiration two conditions are essential; 1. The respiratory organs must have a rich blood supply with very thin moist epithelium covering the blood vessels so that these blood vessels are through into close contact with the environment (water or air). 2. The organs of respiration the blood vessels should be reduced to thin capillaries which expose a large surface area to the environment, so that blood is brought into close contact with the water or air in the respiratory organs. Gills Cartilaginous fishes: Septal gills. Cartilaginous fishes (Sharks) use gills to breathe rather than lungs. There is 5 to 7 gill arches, each bearing one gill slit and covered by the operculum, which acts as a lid over the gill. -
Bodies and Systems
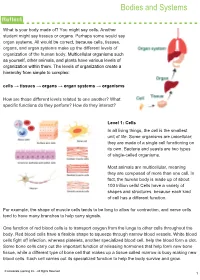
Bodies and Systems What is your body made of? You might say cells. Another student might say tissues or organs. Perhaps some would say organ systems. All would be correct, because cells, tissues, organs, and organ systems make up the different levels of organization of the human body. Multicellular organisms such as yourself, other animals, and plants have various levels of organization within them. The levels of organization create a hierarchy from simple to complex: cells → tissues → organs → organ systems → organisms How are those different levels related to one another? What specific functions do they perform? How do they interact? Level 1: Cells In all living things, the cell is the smallest unit of life. Some organisms are unicellular; they are made of a single cell functioning on its own. Bacteria and yeasts are two types of single-celled organisms. Most animals are multicellular, meaning they are composed of more than one cell. In fact, the human body is made up of about 100 trillion cells! Cells have a variety of shapes and structures, because each kind of cell has a different function. For example, the shape of muscle cells tends to be long to allow for contraction, and nerve cells tend to have many branches to help carry signals. One function of red blood cells is to transport oxygen from the lungs to other cells throughout the body. Red blood cells have a flexible shape to squeeze through narrow blood vessels. White blood cells fight off infection, whereas platelets, another specialized blood cell, help the blood form a clot. -

Mammalian Organogenesis in Deep Time: Tools for Teaching and Outreach Marcelo R
Sánchez‑Villagra and Werneburg Evo Edu Outreach (2016) 9:11 DOI 10.1186/s12052-016-0062-y REVIEW Open Access Mammalian organogenesis in deep time: tools for teaching and outreach Marcelo R. Sánchez‑Villagra1 and Ingmar Werneburg1,2,3,4* Abstract Mammals constitute a rich subject of study on evolution and development and provide model organisms for experi‑ mental investigations. They can serve to illustrate how ontogeny and phylogeny can be studied together and how the reconstruction of ancestors of our own evolutionary lineage can be approached. Likewise, mammals can be used to promote ’tree thinking’ and can provide an organismal appreciation of evolutionary changes. This subject is suitable for the classroom and to the public at large given the interest and familiarity of people with mammals and their closest relatives. We present a simple exercise in which embryonic development is presented as a transforma‑ tive process that can be observed, compared, and analyzed. In addition, we provide and discuss a freely available animation on organogenesis and life history evolution in mammals. An evolutionary tree can be the best tool to order and understand those transformations for different species. A simple exercise introduces the subject of changes in developmental timing or heterochrony and its importance in evolution. The developmental perspective is relevant in teaching and outreach efforts for the understanding of evolutionary theory today. Keywords: Development, Ontogeny, Embryology, Phylogeny, Heterochrony, Recapitulation, Placentalia, Human Background (Gilbert 2013), followed by the growth process. In pla- Mammals are a diverse group in which to examine devel- cental mammals, organogenesis takes place mostly in the opment and evolution, and besides the mouse and the rat uterus, whereas in monotremes and marsupials a very used in biomedical research, provide subjects based on immature hatchling or newborn, respectively, develops which experimental (Harjunmaa et al. -

Experimental Translocation of the Eastern Tiger Salamander in New Jersey: a Conservation Success Story
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/329104272 Experimental Translocation of the Eastern Tiger Salamander in New Jersey: A Conservation Success Story Technical Report · November 2018 CITATIONS READS 0 3 1 author: Robert T. Zappalorti Herpetological Associates, Inc. Wildlife Consultants, 405 Magnolia Road, Pemberton, NJ 08068 84 PUBLICATIONS 722 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Hi Walt. Radio-tracking Pines, Corns and a few Rattlesnakes in the Pines. View project All content following this page was uploaded by Robert T. Zappalorti on 21 November 2018. The user has requested enhancement of the downloaded file. Experimental Translocation of the Eastern Tiger Salamander in New Jersey: A Conservation Success Story Adult male Eastern Tiger Salamander Submitted - November 18, 2018 to David M. Golden, Assistant Director New Jersey Division of Fish and Wildlife PO Box 420, Trenton, New Jersey 08625 By Robert T. Zappalorti Herpetological Associates, Inc. Environmental Consultants 405 Magnolia Road, Pemberton, New Jersey 08068 A publication of the Zappalorti Institute for Pinelands Research Visit our web site @: HerpetologicalAssociates.com Experimental Translocation of the Eastern Tiger Salamander in New Jersey: A Conservation Success Story Introduction Amphibian declines has been an ongoing concern in the scientific community (Lannoo, 2005). Historically, the eastern tiger salamander’s (Ambystoma tigrinum), range originally encompassed 8 different counties in southern New Jersey. In 2018, tiger salamanders are now restricted to only 3 southern New Jersey counties (e.g., Cape May, Cumberland and Salem). Within these three counties the remaining populations are known to successfully breed at approximately 13 suitable wetland locations. -

Introductory Guide Meddra Version 21.0
Introductory Guide MedDRA Version 21.0 March 2018 000148 Notice to Reader Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory Guides have been developed to support languages other than English and are included with their specific translation copies. The Introductory Guide is intended for use in conjunction with the MedDRA Browsers, available with each MedDRA subscription. Changes which are version specific or changes in documentation may be found in the What’s New document. This document is included with the MedDRA release and is also posted on the MSSO Web site under Support Documentation. The MedDRA terminology is maintained under an ISO 9001:2008 registered quality management system. Changes of note in the MedDRA Introductory Guide Version 21.0 include: • Section 4.8 BODY SITE CONSIDERATIONS Additional explanation is provided to support that in general, the abdominal wall is classified in MedDRA as a gastrointestinal structure. • Appendix B: MedDRA CONCEPT DESCRIPTIONS: New Concept Description Product storage has been added Existing Concept Description Medication error has been updated MedDRA Introductory Guide Version 21.0 ii March 2018 000148 Acknowledgements Acknowledgements MedDRA® trademark is registered by IFPMA on behalf of ICH. The following sources of information are also acknowledged: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) Copyright 2013 American Psychiatric Association. ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification, Copyright 1998 Medicode, Inc. COSTART Thesaurus Fifth Edition, Copyright 1995 US Food and Drug Administration (FDA). Hoechst Adverse Reaction Terminology System (HARTS), Copyright 1992 Aventis Pharma.