Evaluating the Water Quality Within the Reticulation System of Kwekwe Municipality During the Dry and Wet Seasons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Midlands Province Mobile Voter Registration Centres
Midlands Province Mobile Voter Registration Centres Chirumhanzu District Team 1 Ward Centre Dates 18 Mwire primary school 10/06/13-11/06/13 18 Tokwe 4 clinic 12/06/13-13/06/13 18 Chingegomo primary school 14/06/13-15/06/13 16 Chishuku Seondary school 16/06/13-18/06/13 9 Upfumba Secondary school 19/06/13-21/06/13 3 Mutya primary school 22/06/13-24/06/13 2 Gonawapotera secondary school 25/06/13-27/06/13 20 Wildegroove primary school 28/06/13-29/06/13 15 Kushinga primary school 30/06/13-02/07/13 12 Huchu compound 03/07/13-04/-07/13 12 Central estates HQ 5/7/13 20 Mtao/Fair Field compound 6/7/13 12 Chiudza homestead 07/07/13-08/06/13 14 Njerere primary school 9/7/13 Team 2 Ward Centre Dates 22 Hillview Secondary school 10/07/13-12/07/13 17 Lalapanzi Secondary school 13/07/13-15/07/13 16 Makuti homestead 16/06/13-17/06/13 1 Mapiravana Secondary school 18/06/13-19/06/13 9 Siyahukwe Secondary school 20/06/13-23/06/13 4 Chizvinire primary school 24/06/13-25/06/13 21 Mukomberana Seconadry school 26/06/13-29/06/13 20 Union primary school 30/06/13-01/07/13 15 Nyikavanhu primary school 02/07/13-03/07/13 19 Musens primary school 04/07/13-06/07/13 16 Utah primary school 07/7/13-09/07/13 Team 3 Ward Centre Dates 11 Faerdan primary school 10/07/13-11/07/13 11 Chamakanda Secondary school 12/07/13-14/07/13 11 Chamakanda primary school 15/07/13-16/07/13 5 Chizhou Secondary school 17/06/13-16/06/13 3 Chilimanzi primary school 21/06/13-23/06/13 25 Maponda primary school 24/06/13-25/06/13 6 Holy Cross seconadry school 26/06/13-28/06/13 20 New England Secondary -
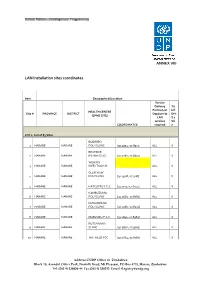
LAN Installation Sites Coordinates
ANNEX VIII LAN Installation sites coordinates Item Geographical/Location Service Delivery Tic Points (List k if HEALTH CENTRE Site # PROVINCE DISTRICT Dept/umits DHI (EPMS SITE) LAN S 2 services Sit COORDINATES required e LOT 1: List of 83 Sites BUDIRIRO 1 HARARE HARARE POLYCLINIC [30.9354,-17.8912] ALL X BEATRICE 2 HARARE HARARE RD.INFECTIO [31.0282,-17.8601] ALL X WILKINS 3 HARARE HARARE INFECTIOUS H ALL X GLEN VIEW 4 HARARE HARARE POLYCLINIC [30.9508,-17.908] ALL X 5 HARARE HARARE HATCLIFFE P.C.C. [31.1075,-17.6974] ALL X KAMBUZUMA 6 HARARE HARARE POLYCLINIC [30.9683,-17.8581] ALL X KUWADZANA 7 HARARE HARARE POLYCLINIC [30.9285,-17.8323] ALL X 8 HARARE HARARE MABVUKU P.C.C. [31.1841,-17.8389] ALL X RUTSANANA 9 HARARE HARARE CLINIC [30.9861,-17.9065] ALL X 10 HARARE HARARE HATFIELD PCC [31.0864,-17.8787] ALL X Address UNDP Office in Zimbabwe Block 10, Arundel Office Park, Norfolk Road, Mt Pleasant, PO Box 4775, Harare, Zimbabwe Tel: (263 4) 338836-44 Fax:(263 4) 338292 Email: [email protected] NEWLANDS 11 HARARE HARARE CLINIC ALL X SEKE SOUTH 12 HARARE CHITUNGWIZA CLINIC [31.0763,-18.0314] ALL X SEKE NORTH 13 HARARE CHITUNGWIZA CLINIC [31.0943,-18.0152] ALL X 14 HARARE CHITUNGWIZA ST.MARYS CLINIC [31.0427,-17.9947] ALL X 15 HARARE CHITUNGWIZA ZENGEZA CLINIC [31.0582,-18.0066] ALL X CHITUNGWIZA CENTRAL 16 HARARE CHITUNGWIZA HOSPITAL [31.0628,-18.0176] ALL X HARARE CENTRAL 17 HARARE HARARE HOSPITAL [31.0128,-17.8609] ALL X PARIRENYATWA CENTRAL 18 HARARE HARARE HOSPITAL [30.0433,-17.8122] ALL X MURAMBINDA [31.65555953980,- 19 MANICALAND -

Midlands Province
School Province District School Name School Address Level Primary Midlands Chirumanzu BARU KUSHINGA PRIMARY BARU KUSHINGA VILLAGE 48 CENTAL ESTATES Primary Midlands Chirumanzu BUSH PARK MUSENA RESETTLEMENT AREA VILLAGE 1 MUSENA Primary Midlands Chirumanzu BUSH PARK 2 VILLAGE 5 WARD 19 CHIRUMANZU Primary Midlands Chirumanzu CAMBRAI ST MATHIAS LALAPANZI TOWNSHIP CHIRUMANZU Primary Midlands Chirumanzu CHAKA NDARUZA VILLAGE HEAD CHAKA Primary Midlands Chirumanzu CHAKASTEAD FENALI VILLAGE NYOMBI SIDING Primary Midlands Chirumanzu CHAMAKANDA TAKAWIRA RESETTLEMENT SCHEME MVUMA Primary Midlands Chirumanzu CHAPWANYA HWATA-HOLYCROSS ROAD RUDUMA VILLAGE Primary Midlands Chirumanzu CHIHOSHO MATARITANO VILLAGE HEADMAN DEBWE Primary Midlands Chirumanzu CHILIMANZI NYONGA VILLAGE CHIEF CHIRUMANZU Primary Midlands Chirumanzu CHIMBINDI CHIMBINDI VILLAGE WARD 5 CHIRUMANZU Primary Midlands Chirumanzu CHINGEGOMO WARD 18 TOKWE 4 VILLAGE 16 CHIRUMANZU Primary Midlands Chirumanzu CHINYUNI CHINYUNI WARD 7 CHUKUCHA VILLAGE Primary Midlands Chirumanzu CHIRAYA (WYLDERGROOVE) MVUMA HARARE ROAD WASR 20 VILLAGE 1 Primary Midlands Chirumanzu CHISHUKU CHISHUKU VILAGE 3 CHIEF CHIRUMANZU Primary Midlands Chirumanzu CHITENDERANO TAKAWIRA RESETTLEMENT AREA WARD 11 Primary Midlands Chirumanzu CHIWESHE PONDIWA VILLAGE MAPIRAVANA Primary Midlands Chirumanzu CHIWODZA CHIWODZA RESETTLEMENT AREA Primary Midlands Chirumanzu CHIWODZA NO 2 VILLAGE 66 CHIWODZA CENTRAL ESTATES Primary Midlands Chirumanzu CHIZVINIRE CHIZVINIRE PRIMARY SCHOOL RAMBANAPASI VILLAGE WARD 4 Primary Midlands -

School Level Province District School Name School Address Secondary
School Level Province District School Name School Address Secondary Midlands Chirumanzu CHAMAKANDA LYNWOOD CENTER TAKAWIRA RESETTLEMENT Secondary Midlands Chirumanzu CHENGWENA RAMBANAPASI VILLAGE, CHIEF HAMA CHIRUMANZU Secondary Midlands Chirumanzu CHISHUKU VILLAGE 2A CHISHUKU RESETLEMENT Secondary Midlands Chirumanzu CHIVONA DENHERE VILLAGE WARD 3 MHENDE CMZ Secondary Midlands Chirumanzu CHIWODZA VILLAGE 38 CHIWODZA RESETTLEMENT MVUMA Secondary Midlands Chirumanzu CHIZHOU WARD 5 MUZEZA VILLAGE, HEADMAN BANGURE , CHIRUMANZU Secondary Midlands Chirumanzu DANNY DANNY SEC Secondary Midlands Chirumanzu DRIEFONTEIN DRIEFONTEIN MISSION FARM Secondary Midlands Chirumanzu GONAWAPOTERA CHAKA BUSINESS CENTRE MVUMA MASVINGO ROAD Secondary Midlands Chirumanzu HILLVIEW HILLVIEW VILLAGE1, LALAPANZI Secondary Midlands Chirumanzu HOLY CROSS HOLY CROSS MISSION WARD 6 CHIRUMANZU Secondary Midlands Chirumanzu LALAPANZI 42KM ALONG GWERU-MVUMA ROAD Secondary Midlands Chirumanzu LEOPOLD TAKAWIRA LEOPOLD TAKAWIRA 2KM ALONG CENTRAL ESTATES ROAD Secondary Midlands Chirumanzu MAPIRAVANA MAPIRAVANA VILLAGE WARD 1CHIRUMANZU Secondary Midlands Chirumanzu MUKOMBERANWA MUWANI VILLAGE HEADMAN MANHOVO Secondary Midlands Chirumanzu MUSENA VILLAGE 8 MUSENA RESETTLEMENT Secondary Midlands Chirumanzu MUSHANDIRAPAMWE RUDHUMA VILLAGE WARD 25 CHIRUMANZU Secondary Midlands Chirumanzu MUTENDERENDE DZORO VILLAGE CHIEF HAMA Secondary Midlands Chirumanzu NEW ENGLAND LOVEDALE FARMSUB-DIVISION 2 MVUMA Secondary Midlands Chirumanzu ORTON'S DRIFT ORTON'S DRIFT FARM Secondary Midlands -

Midlands North Inspection Stations
MIDLANDS NORTH INSPECTION STATIONS GOKWE CENTRAL 1 BHEJANI PRIMARY SCHOOL 2 CHEVECHEVE SECONDARY SCHOOL 3 CHIDAMOYO PRIMARY SCHOOL 4 CHIDOMA PRIMARY SCHOOL 5 CHITAPO BUSINESS CENTRE 6 GANYUNGU PRIMARY SCHOOL 7 GWANYIKA PRIMARY SCHOOL 8 GWENUNGU PRIMARY SCHOOL 9 GWENYA PRIMARY SCHOOL 10 JAHANA PRIMARY SCHOOL 11 KASIKANA PRIMARY SCHOOL 12 KRIMA PRIMARY SCHOOL 13 MALIYAMI PRIMARY SCHOOL 14 MAPU PRIMARY SCHOOL 15 MTANKI PRIMARY SCHOOL 16 MUTANGE C.B. PRIMARY SCHOOL 17 MWEMBESI PRIMARY SCHOOL 18 NDABAMBI PRIMARY SCHOOL 19 NHONGO PRIMARY SCHOOL 20 SATENGWE PRIMARY SCHOOL 21 SENGWA PRIMARY SCHOOL 22 SIMBE PRIMARY SCHOOL 23 ST. CUTHBERTS MASORO PRIMARY SCHOOL 24 TONGWE SECONDARY SCHOOL 25 CHIZIYA COMMUNITY HALL 26 GABABE PRIMARY SCHOOL 27 RAJI MINISTRY OF TRANSPORT 28 GWEHAVA PRIMARY SCHOOL GOKWE EAST 1 BATSIRAI PRIMARY SCHOOL 2 CHAMINUKA PRIMARY SCHOOL 3 CHINYENYETU PRIMARY SCHOOL 4 CHIODZA PRIMARY SCHOOL 5 COPPER QUEEN BUSINESS CENTRE 6 DENDA PRIMARY SCHOOL 7 DEWE PRIMARY SCHOOL 8 DINDIMUTIWI PRIMARY SCHOOL 9 GANDAVACHE PRIMARY SCHOOL 10 GOREDEMA PRIMARY SCHOOL 11 GWEBO PRIMARY SCHOOL 12 HWADZE PRIMARY SCHOOL 13 KAHOBO PRIMARY SCHOOL 14 KAMWA PRIMARY SCHOOL 15 KAMWAMBE PRIMARY SCHOOL 16 KASONDE PRIMARY SCHOOL 17 KUEDZA PRIMARY SCHOOL 18 KWAEDZA PRIMARY SCHOOL 19 MADZIDRIRE PRIMARY SCHOOL 20 MASOSONI PRIMARY SCHOOL 21 MHUMHA PRIMARY SCHOOL 22 MSADZI PRIMARY SCHOOL 23 MUDONDO PRIMARY SCHOOL 24 MUPAWA PRIMARY SCHOOL 25 MUSOROWENZOU PRIMARY SCHOOL 26 MUTEHWE PRIMARY SCHOOL 27 MUTUKANYI PRIMARY SCHOOL 28 NORAH PRIMARY SCHOOL 29 NYAMAZENGWE PRIMARY -

The Struggle for Democracy in the Political Minefield of Zimbabwe
THE STRUGGLE FOR DEMOCRACY IN THE POLITICAL MINEFIELD OF ZIMBABWE A STORY OF THE POLITICAL VIOLENCE EXPERIENCED BY BLESSING CHEBUNDO, MEMBER OF PARLIAMENT, KWEKWE MOVEMENT FOR DEMOCRATIC CHANGE, ZIMBABWE Julius Caesar wrote: “I came, I saw, I conquered”. And I say: “I entered the Zimbabwe Political arena, I fight for Democracy, I will continue the struggle”. My story starts with the Zimbabwe Constitutional Referendum, held on the 12th February 2000, which saw President Robert Mugabe’s Zanu PF getting its first national defeat in the Political Arena and thereby setting the tone for Zimbabwe’s political violence. Sensing danger of a political whitewash by the newly formed MDC, Zanu PF gathered all its violent political might to crush the young MDC Party and its supporters. By voting against the changes in the Zimbabwe Constitutional Referendum, the people of Zimbabwe had taken heed of the call by the combined efforts of the MDC and the National Constitutional Assembly (NCA) and had demonstrated their intolerance to the misrule of the prior 20 years by Zanu PF. As is 1 already known, the first targets were the white commercial farmers, their workers and the MDC activists. The whirlwind of political violence began with an opening bang in February 2000!! I had worked with Paul Themba Nyathi both under the NCA and since the inception of the MDC, during the peoples pre-convention. Pre-convention is the period for intensive coordination of Civic Society organisations leading to the birth of the MDC. Paul was a member of the MDC’s interim National Executive Committee (NEC), whilst I was the Interim Provincial Chairman for Midlands North. -

Are They Accountable? Examining Alleged Violators and Their Violations Pre and Post the Presidential Election March 2002
[report also available from: http://www.hrforumzim.com ] ZIMBABWE HUMAN RIGHTS NGO FORUM Are they accountable? Examining alleged violators and their violations pre and post the Presidential Election March 2002 A report by the Zimbabwe Human Rights NGO Forum December 2002 Zimbabwe Human Rights NGO Forum Are They Accountable? The Zimbabwe Human Rights NGO Forum (also known as the “Human Rights Forum”) has been in existence since January 1998. Nine non-governmental organisations working in the field of human rights joined together to provide legal and psychosocial assistance to the victims of the Food Riots of January 1998. The Human Rights Forum has now expanded its objectives to assist victims of organised violence, using the following definition: “Organised violence” means the inter-human infliction of significant avoidable pain and suffering by an organised group according to a declared or implied strategy and/or system of ideas and attitudes. It comprises any violent action, which is unacceptable by general human standards, and relates to the victims’ mental and physical well-being.” The Human Rights Forum operates a Legal Unit and a Research and Documentation Unit. Core member organisations of the Human Rights Forum are: · Amani Trust · Amnesty International (Zimbabwe) (AI (Z)) · Catholic Commission for Justice and Peace (CCJP) · Gays and Lesbians of Zimbabwe (GALZ) · Legal Resources Foundation (LRF) · Transparency International (Zimbabwe) (TI (Z)) · University of Zimbabwe Legal Aid and Advice Scheme · Zimbabwe Association for Crime Prevention and the Rehabilitation of the Offender (ZACRO) · Zimbabwe Civic Education Trust (ZIMCET) · Zimbabwe Human Rights Association (ZimRights) · Zimbabwe Lawyers for Human Rights (ZLHR) · Zimbabwe Women Lawyers Association (ZWLA) Associate Member: · Nonviolent Action and Strategies for Social Change (NOVASC) The Human Rights Forum can be contacted through any member organisation or through: 1. -

Preventing Electoral Fraud in Zimbabwe
Have you ever heard of a voters' roll with large numbers of 110-year-olds on it, all born on the same day? Or a small African country whose electorate contains over 40,000 centenarians - and not a few child voters, some of them as young as two years old? Even more remarkable, the country in question, Zimbabwe, has lost about four million of its citizens to the diaspora, as people have fled the results of President Robert Mugabe's ruinous rule – and yet the total electorate is defying gravity and continuing to grow strongly. Welcome to the Alice-in-Wonderland world of the Zimbabwean voters' roll....... In this Report, R.W. Johnson shows just how thorough electoral reform needs to be in Zimbabwe if that country is to see free and fair elections in the future. When R.W. Johnson was Director of the Helen Suzman Foundation in Johannes- burg, he carried out ground-breaking research in Zimbabwe. In particular, he conducted the first systematic political opinion surveys there from the late 1990s to the early years of the 21st Century. These surveys showed conclusively that, from 2000 on, the Mugabe regime had lost the support of the majority of voters. Johnson’s surveys received world-wide recognition and did not endear him to the Mugabe regime. So much so that he was forced to work there under-cover and was once able to watch a half-hour Zimbabwe Television programme all about himself as "the evil genius behind Zimbabwe's political discontent". Zimbabwe's discontents have hardly lessened; and this Report continues the work that was begun more than a decade ago. -
Maria Ward Schwestern in SIMBABWE
Maria Ward-Schwestern CONGREGATIO JESU SIMBABWE soziale pastorale Dienste Kindergarten Kinderheim Schulen amb. Kliniken MARIA WARD SCHWESTERN seit 1951 in Simbabwe 1 AMAVENI Vorschule Die Vorschule von Amaveni wird von ca. 80 Kindern besucht. Davon sind etwa 7 Kinder aus dem Children’s Home Amaveni. Es ist uns möglich, monatlich einen Beitrag für die entstehenden Kosten zu senden von etwa 500 Euro. 2 AMAVENI Children’s Home Im Children’s Home leben in 4 Häusern jeweils ca. 17 Kinder und Schülerinnen/Schüler. In jedem Haus ist immer eine Hausmutter zuständig. Die Kinder/Jugendlichen jeder Hausgemeinschaft verstehen sich als Geschwister; sie werden betreut von jeweils 2 Hausmüttern, die etwa 8 Stunden im Wechsel für sie da sind; die übrige Zeit teilen sich die Schwestern. Die Schülerinnen und Schüler besuchen: Vorschule, Grundschule, Sekundarschule, College, Universität z. T. mit Internatskosten. Unser derzeit jüngstes Kind ist Tinashe EMMANUEL, hier kurz nach der Geburt im November 2020, zusammen mit der Leiterin des Kinderheimes, Sr. Aleta Dube CJ. Die Kinder und Ju- gendlichen im Child- ren’s Home verdienen tatkräftige Unterstüt- zung, damit sie in Wür- de und frei von Furcht und Not leben können. Es ist unser Ziel, dass sie einst als selbstständige Men- schen ihre Zukunft meistern können. 3 SCHÜLER AMAVENI Wir sind sehr froh, dass wir für das Children’s Home viele Freunde und Förderer haben und sind froh, wenn noch weitere hinzukommen. Unser monatlicher Beitrag für das Kinderheim beträgt: 9.600 €. Derzeit sparen wir für die Dachreparaturen der 4 Häuser, sie sind leider nicht mehr regensicher. Schülerinnen/Schüler besuchen Vorschule, Grundschule, Sekundarschule, College, Universität, Ausbildungsstätten. -

Midlands Province
Page 1 of 33 Midlands Province LOCAL AUTHORITY NAME OF CONSTITUENCY WARDNUMBER NAME OF POLLING STATION FACILITY Takawira RDC Chirumanzu 1 Mapirivana Secondary School Takawira RDC Chirumanzu 1 Magada Primary School Takawira RDC Chirumanzu 1 Chiweshe A Primary School Takawira RDC Chirumanzu 1 Chiweshe B Primary School Takawira RDC Chirumanzu 1 Majandu Primary School Takawira RDC Chirumanzu 1 Mapirivana Primary School 6 Takawira RDC Chirumanzu 2 Gonawapotera A Secondary school Takawira RDC Chirumanzu 2 Gonawapotera B Secondary school Takawira RDC Chirumanzu 2 Chaka Health centre Takawira RDC Chirumanzu 2 Makanya A Primary School Takawira RDC Chirumanzu 2 Makanya B Primary School 5 Takawira RDC Chirumanzu 3 Vudzi Primary School Takawira RDC Chirumanzu 3 Mutya Primary School Takawira RDC Chirumanzu 3 Chilimanzi A Primary School Takawira RDC Chirumanzu 3 Chilimanzi B Primary School Takawira RDC Chirumanzu 3 Mhende Primary School Takawira RDC Chirimanzu 13 Chivona Secondary School 6 Takawira RDC Chirumanzu 4 Chingwena Secondary school Takawira RDC Chirumanzu 4 Juru BC Tent Takawira RDC Chirumanzu 4 Chengwena Health centre Takawira RDC Chirumanzu 4 Chizvinire Primary School 4 Takawira RDC Chirumanzu 5 Chizhou Secondary school Takawira RDC Chirumanzu 5 Nhomboka Primary School Takawira RDC Chirumanzu 5 Chimbindi Primary School Takawira RDC Chirumanzu 5 Muzeza Primary School Chirumanzu 5 Chizhou 5 Takawira RDC Chirumanzu 6 Holy Cross Secondary school Takawira RDC Chirumanzu 6 Chipenzi BC Tent Takawira RDC Chirumanzu 6 Holy Cross BC Tent Takawira -

Parliamentary Elections 2005 Polling Stations Midlands
PARLIAMENTARY ELECTIONS 2005 POLLING STATIONS MIDLANDS PROVINCE As published in The Zimbabwe Independent dated March 18, 2005 Polling stations shall be open from 0700 hours to 1900 hours on polling day CHIRUMANZU CONSTITUENCY 1 Chizvinire Primary School 2 Chinyuni Primary School 3 Shashe Primary School 4 Mutenderende Secondary School 5 Mashamba Primary School 6 Maware Primary School 7 Chengwena Secondary School 8 Chiona Secondary School 9 Mhende Primary School 10 Ngezi Primary School 11 Siyahokwe Secondary School 12 Chilimanzi Primary School 13 Mutya Primary School 14 Mavhaire Primary School 15 Chihosho Primary School 16 Vhudzi Primary School 17 Rupepwe Primary School 18 Taringana Secondary School 19 Charandura Council Hall 20 Govere Primary School 21 Bhoroma (Gonamombe) Centre 22 Chapwanya Primary School 23 Maponda Primary School 24 Debwe Primary School 25 Guramatunhu Primary School 26 Nyautonge Primary School 27 Rutunga Primary School 28 Hwata Primary School 29 Chimbindi Primary School 30 Nhomboka Primary School 31 Majandu Primary School 32 Magada Primary School 33 Chizhou Secondary School 34 Muzeza Primary School 35 Chiwesha Primary School 36 Munanzvi Business Centre 37 Holycross Secondary School 38 Makanya Primary School 39 Gambiza Primary School 40 Nyamandi Primary School 41 Mazvimba Primary School 42 Muwani Primary School 43 Gonawapotera Secondary School 44 Mukomberanwa Secondary School 45 Mapiravana Secondary School 46 Mapiravana Primary School 47 Tokwe 4 Clinic 48 Chingegomo Primary School 49 Mwire Primary School 50 Lalapanzi Secondary -

MIDLANDS PROVINCE - Basemap
MIDLANDS PROVINCE - Basemap Mashonaland West Chipango Charara Dunga Kazangarare Charara Hewiyai 8 18 Bvochora 19 9 Ketsanga Gota Lynx Lynx Mtoranhanga Chibara 2 29 Locations A Chitenje 16 21 R I B 4 K A CHARARA 7 Mpofu 23 K E 1 Kachuta 4 L A 12 SAFARI Kapiri DOMA 1 Kemutamba Mwami Kapiri Chingurunguru SAFARI Kosana Guruve AREA Mwami Kapiri Green Matsvitsi Province Capital Mwami Mwami Bakwa AREA Valley 5 Guruve 6 KARIBA 26 Kachiva 1 Doro Shinje Nyaodza Dora Kachiva Shinje Ruyamuro B A R I Masanga Nyamupfukudza 22 Nyama A Nyamupfukudza C e c i l m o u r 22 Town E K Kachekanje Chiwore 18 Nyakapupu K Masanga Doma 2 7 L A Gache- Kachekanje Masanga 23 Lan Doma 3 Gatse Gache lorry 5 Doma Gatse Masanga B l o c k l e y Chipuriro 2 Maumbe Maumbe Rydings GURUVE Bumi 16 Maumbe Hills Gachegache Chikanziwe 8 Kahonde Garangwe Karoi 15 Place of Local Importance Magwizhu Charles Lucky Chalala Tashinga Kareshi Crown 5 Mauya 10 11 Clarke 7 10 3 Mauya Chalala Charles Nyangoma 11 1 Karambanzungu Chitimbe Clarke Magunje 8 Nyamhondoro Hesketh Bepura Chalala Kabvunde KAROI URBAN Mugarakamwe Karambanzungu Magunje Magunje Hesketh Nyangoma Mhangura 9 Kudzanai Army Government Ridziwi Mudindo Nyamhunga Sikona ARDA 9 Mission Nyamhunga Mahororo Charles Chisape HURUNGWE Mhangura Sisi MATUSADONA Murapa Sengwe Dombo Madadzi Nyangoma Mhangura 12 Clack Karoi Katenhe Arda Kanyati Nyamhunga Enterprise Tategura Mhangura NATIONAL 17 Kapare Katenhe Karoi Mine Mhangura Sisi Mola Makande Nyadara Muitubu Mola Nyamhunga 10 Enterprise 14 Mola PARK Makande 11 Mhangura Ramwechete 11