Mussel, Cockle, Variegated Scallop, and Razor Shell) Normally Consumed in Spain, 2005
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Siliqua Patula Class: Bivalvia; Heterodonta Order: Veneroida the Flat Razor Clam Family: Pharidae
Phylum: Mollusca Siliqua patula Class: Bivalvia; Heterodonta Order: Veneroida The flat razor clam Family: Pharidae Taxonomy: The familial designation of this (see Plate 397G, Coan and Valentich-Scott species has changed frequently over time. 2007). Previously in the Solenidae, current intertidal Body: (see Plate 29 Ricketts and Calvin guides include S. patula in the Pharidae (e.g., 1952; Fig 259 Kozloff 1993). Coan and Valentich-Scott 2007). The superfamily Solenacea includes infaunal soft Color: bottom dwelling bivalves and contains the two Interior: (see Fig 5, Pohlo 1963). families: Solenidae and Pharidae (= Exterior: Cultellidae, von Cosel 1993) (Remacha- Byssus: Trivino and Anadon 2006). In 1788, Dixon Gills: described S. patula from specimens collected Shell: The shell in S. patula is thin and with in Alaska (see Range) and Conrad described sharp (i.e., razor-like) edges and a thin profile the same species, under the name Solen (Fig. 4). Thin, long, fragile shell (Ricketts and nuttallii from specimens collected in the Calvin 1952), with gapes at both ends Columbia River in 1838 (Weymouth et al. (Haderlie and Abbott 1980). Shell smooth 1926). These names were later inside and out (Dixon 1789), elongate, rather synonymized, thus known synonyms for cylindrical and the length is about 2.5 times Siliqua patula include Solen nuttallii, the width. Solecurtus nuttallii. Occasionally, researchers Interior: Prominent internal vertical also indicate a subspecific epithet (e.g., rib extending from beak to margin (Haderlie Siliqua siliqua patula) or variations (e.g., and Abbott 1980). Siliqua patula var. nuttallii, based on rib Exterior: Both valves are similar and morphology, see Possible gape at both ends. -

Fish Bulletin 161. California Marine Fish Landings for 1972 and Designated Common Names of Certain Marine Organisms of California
UC San Diego Fish Bulletin Title Fish Bulletin 161. California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California Permalink https://escholarship.org/uc/item/93g734v0 Authors Pinkas, Leo Gates, Doyle E Frey, Herbert W Publication Date 1974 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY OF CALIFORNIA DEPARTMENT OF FISH AND GAME FISH BULLETIN 161 California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California By Leo Pinkas Marine Resources Region and By Doyle E. Gates and Herbert W. Frey > Marine Resources Region 1974 1 Figure 1. Geographical areas used to summarize California Fisheries statistics. 2 3 1. CALIFORNIA MARINE FISH LANDINGS FOR 1972 LEO PINKAS Marine Resources Region 1.1. INTRODUCTION The protection, propagation, and wise utilization of California's living marine resources (established as common property by statute, Section 1600, Fish and Game Code) is dependent upon the welding of biological, environment- al, economic, and sociological factors. Fundamental to each of these factors, as well as the entire management pro- cess, are harvest records. The California Department of Fish and Game began gathering commercial fisheries land- ing data in 1916. Commercial fish catches were first published in 1929 for the years 1926 and 1927. This report, the 32nd in the landing series, is for the calendar year 1972. It summarizes commercial fishing activities in marine as well as fresh waters and includes the catches of the sportfishing partyboat fleet. Preliminary landing data are published annually in the circular series which also enumerates certain fishery products produced from the catch. -

Os Nomes Galegos Dos Moluscos 2020 2ª Ed
Os nomes galegos dos moluscos 2020 2ª ed. Citación recomendada / Recommended citation: A Chave (20202): Os nomes galegos dos moluscos. Xinzo de Limia (Ourense): A Chave. https://www.achave.ga /wp!content/up oads/achave_osnomesga egosdos"mo uscos"2020.pd# Fotografía: caramuxos riscados (Phorcus lineatus ). Autor: David Vilasís. $sta o%ra est& su'eita a unha licenza Creative Commons de uso a%erto( con reco)ecemento da autor*a e sen o%ra derivada nin usos comerciais. +esumo da licenza: https://creativecommons.org/ icences/%,!nc-nd/-.0/deed.g . Licenza comp eta: https://creativecommons.org/ icences/%,!nc-nd/-.0/ ega code. anguages. 1 Notas introdutorias O que cont!n este documento Neste recurso léxico fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas) ! primeira edición d" Os nomes galegos dos moluscos é do ano #$%& Na segunda edición (2$#$), adicionáronse algunhas especies, asignáronse con maior precisión algunhas das denominacións vernáculas galegas, corrixiuse algunha gralla, rema'uetouse o documento e incorporouse o logo da (have. )n total, achéganse nomes galegos para *$+ especies de moluscos A estrutura )n primeiro lugar preséntase unha clasificación taxonómica 'ue considera as clases, ordes, superfamilias e familias de moluscos !'uí apúntanse, de maneira xeral, os nomes dos moluscos 'ue hai en cada familia ! seguir -

Molluscs Gastropods
Group/Genus/Species Family/Common Name Code SHELL FISHES MOLLUSCS GASTROPODS Dentalium Dentaliidae 4500 D . elephantinum Elephant Tusk Shell 4501 D . javanum 4502 D. aprinum 4503 D. tomlini 4504 D. mannarense 450A D. elpis 450B D. formosum Formosan Tusk Shell 450C Haliotis Haliotidae 4505 H. varia Variable Abalone 4506 H. rufescens Red Abalone 4507 H. clathrata Lovely Abalone 4508 H. diversicolor Variously Coloured Abalone 4509 H. asinina Donkey'S Ear Abalone 450G H. planata Planate Abalone 450H H. squamata Scaly Abalone 450J Cellana Nacellidae 4510 C. radiata radiata Rayed Wheel Limpet 4511 C. radiata cylindrica Rayed Wheel Limpet 4512 C. testudinaria Common Turtle Limpet 4513 Diodora Fissurellidae 4515 D. clathrata Key-Hole Limpets 4516 D. lima 4517 D. funiculata Funiculata Limpet 4518 D. singaporensis Singapore Key-Hole Limpet 4519 D. lentiginosa 451A D. ticaonica 451B D. subquadrata 451C Page 1 of 15 Group/Genus/Species Family/Common Name Code D. pileopsoides 451D Trochus Trochidae 4520 T. radiatus Radiate Top 4521 T. pustulosus 4522 T. stellatus Stellate Trochus 4523 T. histrio 4524 T. maculatus Maculated Top 452A T. niloticus Commercial Top 452B Umbonium Trochidae 4525 U. vestiarium Common Button Top 4526 Turbo Turbinidae 4530 T. marmoratus Great Green Turban 4531 T. intercostalis Ribbed Turban Snail 4532 T. brunneus Brown Pacific Turban 4533 T. argyrostomus Silver-Mouth Turban 4534 T. petholatus Cat'S Eye Turban 453A Nerita Neritidae 4535 N. chamaeleon Chameleon Nerite 4536 N. albicilla Ox-Palate Nerite 4537 N. polita Polished Nerite 4538 N. plicata Plicate Nerite 4539 N. undata Waved Nerite 453E Littorina Littorinidae 4540 L. scabra Rough Periwinkle 4541 L. -

Damage to Razor Clam (Ensis Siliqua and E
The Glasgow Naturalist (online 2020) Volume 27, Part 3 https://doi.org/10.37208/tgn27303 Herring gull (Larus argentatus) damage to razor clam (Ensis siliqua and E. ensis) shells on the Isle of Cumbrae, Scotland P.G. Moore 32 Marine Parade, Millport, Isle of Cumbrae KA28 0EF E-mail: [email protected] Herring gulls (Larus argentatus ) are an opportunist Fig. 1. Right valves of the razor clams Ensis siliqua (top) and species with a catholic diet (Tinbergen, 1953; Hudson & E. ensis (bottom) from Isle of Cumbrae, Scotland. Note Furness, 1988; Ewins et al., 1994; Moore, 2018). They relative sizes and robustness. (Photo: P.G. Moore) have been reported feeding on razor clams (Ensis leei, was harassed by a great black-backed gull (L. marinus) as "E. directus") in the Dutch Wadden Sea (Cadée, 2000; landing alongside it, which was followed by the herring Enners et al., 2018). Witnessing them feeding on gull flying away with the shell. The herring gulls' initial E. siliqua and E. ensis near my house in Millport, Isle of tendency to open shells at the immediate tide edge Cumbrae, Scotland (NS171547) in April 2020 provided would account for sand grains becoming adherent to the opportunity for comparative observations. flesh fragments found remaining stuck to the inside of the recovered empty shells. Both razor clam species are common in sublittoral sand below the adjacent beach at Kames Bay, Millport (Allen, Most empty shells were orientated with their internal 1962; see Holme, 1951 and Fig . 1 herein for surfaces facing up and the majority (96% of E. -
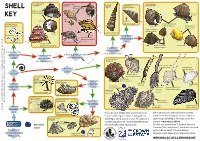
Shell Whelk Dog Whelk Turret It Could Be a Periwinkle Shell (Nucella Lapillus) Shell Spire Shell Thick Top Shell (Osilinus Lineatus) Dark Stripes Key on Body
It could be a type of It could be a type of It could be a It could be a type of topshell whelk Dog whelk turret It could be a periwinkle Shell (Nucella lapillus) shell spire shell Thick top shell (Osilinus lineatus) Dark stripes Key on body Egg Underside capsules Actual size It could be a type of (Hydrobia sp) Common periwinkle spiral worm White ‘Colar’ (Littorina littorea) Flat periwinkle (Littorinasp) Yes Roughly ‘ribbed’ shell. Very high up shore ‘Tooth inside (Turitella communis) opening (Spirorbis sp) Does it have 6 Common whelk No (Buccinum undatum) Yes or more whorls Brown, speckled Netted dog whelk body (twists)? Painted topshell (Nassarius reticulatus) (Calliostoma zizyphinum) No Rough periwinkle Flattened spire Yes Is it long, thin (Littorina saxatilis) Yes Yes and cone shaped Is it permanently No like a unicorn’s horn? attached to Is there a groove or teeth No Is there mother No a surface? in the shell opening? of pearl inside It could be a type of the shell opening? bivalve Yes Yes Common otter-shell (Lutraria lutraria) Bean-like tellin No Is the shell in (Fabulina fabula) Is it 2 parts? spiraled? Common cockle (Cerastoderma edule) It could be a Flat, rounded No sand No Great scallop mason It could be a Is the shell a (Pecten maximus) shell Razor shell worm keel worm Wedge-shaped Is the case dome or (Ensis sp) No Pacific oyster shell made from Yes cone shape? (Crassostrea gigas) Shell can be Peppery furrow shell very large (Scrobicularia plana) sand grains? Elongated and and doesn’t (Lanice conchilega) deep-bodied fully close with large ‘frills’ No (Pomatoceros sp) Yes It could be a type of sea urchin It could be a type of An acorn Native oyster Empty barnacle barnacle Does it have that may be found in estuaries and shores in the UK. -

A Razor Shell (Ensis Ensis)
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles A razor shell (Ensis ensis) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Jacqueline Hill 2006-11-02 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1419]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Hill, J.M. 2006. Ensis ensis A razor shell. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1419.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2006-11-02 A razor shell (Ensis ensis) - Marine Life Information Network See online review for distribution map Ensis ensis, the razor shell. -

(Razor) Clam (Ensis Directus Conrad, 1843) Dale F
NRAC Publication No. 217-2010 University of Maryland, 2113 Animal Science Building College Park, Maryland 20742-2317 Telephone: 301-405-6085, FAX: 301-314-9412 E-mail: [email protected] Web: http://www.nrac.umd.edu Biology of the Atlantic Jacknife (Razor) Clam (Ensis directus Conrad, 1843) Dale F. Leavitt, Roger Williams University, Bristol RI There are many clams that are identified by the com- Anatomy mon name “razor clam”. They gain that moniker due to their overall shape of being long and thin, in the nature The American razor clam is a filter-feeding of an old-time straight razor. Many species of razor bivalve mollusk that is easily recognized due to its clams are favorably recognized for their taste and texture unique shape, where it is 5-8 times longer (anterio-pos- and have been commercially harvested throughout the teriorly) than it is wide and with a shape that describes a world. More recently, interest has been rising about developing methods to farm razor clams. It is important slight arc and does not taper appreciably along its length to understand key aspects of the clam’s basic biology (Figure 1). and natural history before one can start to grow them successfully under controlled conditions. One species that has been identified as having high potential for aquaculture is the Atlantic jack knife or American razor clam, Ensis directus (Figure 1, also identified as Ensis americanus in Europe). A native of the Atlantic seaboard of North America, the Razor clam ranges from Labrador to South Carolina. Our knowledge of this razor clam is somewhat enigmatic given that the bulk of the information on E. -

Species Delimitation and DNA Barcoding of Atlantic Ensis (Bivalvia, Pharidae)
Species delimitation and DNA barcoding of Atlantic Ensis (Bivalvia, Pharidae) Joaquín Viernaa,b, Joël Cuperusc, Andrés Martínez-Lagea, Jeroen M. Jansenc, Alejandra Perinaa,b, Hilde Van Peltc, Ana M. González-Tizóna a Evolutionary Biology Group (GIBE), Department of Molecular and Cell Biology,Universidade da Coruña, A Fraga 10, A Coruña, E-15008, Spain b AllGenetics, Edificio de Servicios Centrales de Investigación, Campus de Elviña s/n, A Coruña, E-15008, Spain c IMARES Wageningen UR, Ambachtsweg 8a, Den Helder, NL-1785 AJ, The Netherlands Zoologica Scripta Volume 43, Issue 2, pages 161–171, March 2014 Issue online: 17 February 2014, Version of record online: 3 September 2013, Manuscript Accepted: 11 August 2013, Manuscript Received: 17 May 2013. This is the peer reviewed version of the following article: Vierna, J., Cuperus, J., Martínez-Lage, A., Jansen, J.M., Perina, A., Van Pelt, H. & González-Tizón, A.M. (2013). Species delimitation and DNA barcoding of Atlantic Ensis (Bivalvia, Pharidae). Zoologica Scripta, 43, 161–171. which has been published in final form at https://doi.org/10.1111/zsc.12038. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. Abstract Ensis Schumacher, 1817 razor shells occur at both sides of the Atlantic and along the Pacific coasts of tropical west America, Peru, and Chile. Many of them are marketed in various regions. However, the absence of clear autapomorphies in the shell and the sympatric distributions of some species often prevent a correct identification of specimens. As a consequence, populations cannot be properly managed, and edible species are almost always mislabelled along the production chain. -

Studies on Shell and Meat Composition of Molluscs from Saurashtra Coast
Studies on shell and meat composition of molluscs from Saurashtra coast Item Type article Authors Sarvaiya, R.T. Download date 25/09/2021 05:48:42 Link to Item http://hdl.handle.net/1834/31726 Journal of the Indian Fisheries Association 19,1989,59-64 STUDIES ON SHELL AND MEAT COMPOSITION OF MOLLUSCS FROM SAURASHTRA COAST R.T. SARVAIYA Office of the Superintendent of Fisheries, Jamnagar-361 005. ABSTRACT Shell composition of 35 species of molluscs was studied. The shell, calcium and calcium carbonate contents were lowest in the cuttle bone of cuttlefish, while they were highest in shell of Cypraea ocel/ata. Solen sp. had higher meat and protein content than the pelecypods and lower meat, water and protein content than the cephalopods. Inverse relationships between water and fat, and protein and ash were established. INTRODUCTION In most of the molluscs, the shell forms a large percentage of body weight. If the molluscan fisheries are to be economically viable, profitable uses for the shells have to be found besides the meat which generally commands fairly attractive prices. It was therefore, considered important to estimate calcium and calcium carbonate in the shells of various species of molluscs. This perhaps may help the number of calcium based small or medium scale industries in Gujarat. Gokhale (1960) has indicated the use of window pane oyster for obtaining calcium. The marine edible razor shell, Solen sp. (Fam Solenidae) occurs throughout the year in good quantity between the inter tidal areas in the Gulf of Kutch, particularly on the northern side of Gopi (lat. -

Appendix 10.1 Commercial Fisheries Technical Report.Pdf
Commercial Fisheries Technical Report Poseidon Aquatic Resource Management Ltd. March 2018 Commercial Fisheries Appendix 10.1 Technical Report Table of Contents 1 Introduction ................................................................................................... 7 1.1 Context ......................................................................................................................... 7 1.2 Commercial Fisheries Study Area .................................................................................. 7 1.3 Data Sources ................................................................................................................. 7 2 Eastern Scottish Ports ................................................................................... 10 2.1 Overview .................................................................................................................... 10 2.2 Fife ............................................................................................................................. 10 2.3 East Lothian and Scottish Borders................................................................................ 14 2.4 Aberdeenshire and Angus ........................................................................................... 18 3 Key Fleets and Fisheries................................................................................ 23 3.1 Overview of Regional Landings .................................................................................... 23 3.2 Key Species ................................................................................................................ -

Validation of a Method for Age Determination in the Razor Clam Ensis Directus with a Review on Available Data on Growth, Reproduction and Physiology
Validation of a method for age determination in the razor clam Ensis directus with a review on available data on growth, reproduction and physiology Joana F.M.F. Cardoso, Gerard Nieuwland, Jeroen Wijsman, Rob Witbaard, Henk W. van der Veer NIOZ Royal Netherlands Institute for Sea Research Validation of a method for age determination in the razor clam Ensis directus with a review on available data on growth, reproduction and physiology Joana F.M.F. Cardoso1, Gerard Nieuwland1, Jeroen Wijsman2, Rob Witbaard1 and Henk W. van der Veer1 1 NIOZ, Postbus 59, 1790AB Den Burg Texel 2 IMARES, Postbus 77, 4400AB Yerseke Commissioned by: Waterdienst Rijkswaterstaat Colophon Report number: 2011-9 Date: October 2011 Published by: NIOZ Netherlands Institute for Sea Research Authors: Joana F.M.F. Cardoso, Gerard Nieuwland, Jeroen Wijsman, Rob Witbaard and Henk W. van der Veer Title: Validation of a method for age determination in the razor clam Ensis directus, with a review on available data on growth, reproduction and physiology Project name: Bepaling van de Dynamic Energy Budget (DEB) parameters van Ensis directus Commissioned by: Rijkswaterstaat Waterdienst Postbus 17 8200 AA Lelystad Programme: Monitoring and Evaluation Programme Sandminning RWS LaMER Part B2 and B4 of the evaluation programme Sandminning Supervised by: Marcel Rozemeijer (RWS-WD) Saa H. Kabuta (RWS-WD) Johan de Kok (Deltares) Rik Duijts (RWS-DNZ) Executed by: NIOZ Netherlands Institute for Sea Research Landsdiep 4 1797 SZ Den Hoorn (Texel) IMARES, Wageningen UR P.O. Box 77 4400 AB Yerseke Tasks: Collection of calcium carbonate powder – Gerard Nieuwland Lab work organization and shell collection – Rob Witbaard Data analysis and report writing – Joana Cardoso Length-weight relationships – Jeroen Wijsman Project management - Jeroen Wijsman and Henk v.d.