DISSERTATION PROTEOMIC PROFILING of the RAT RENAL PROXIMAL CONVOLUTED TUBULE in RESPONSE to CHRONIC METABOLIC ACIDOSIS Submitted
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Grhpr Gene, Full Gene Analysis
TEST ID: GRHPZ GRHPR GENE, FULL GENE ANALYSIS CLINICAL INFORMATION MOBILE APPS FROM MAYO MEDICAL LABORATORIES Primary hyperoxaluria type 2 (PH2) is a hereditary disorder of glyoxylate metabolism caused by deficiency of the hepatic enzyme glyoxylate reductase/hydroxypyruvate reductase (GRHPR). Lab Catalog for iPad and Absence of GRHPR activity results in excess oxalate and usually L-glycerate excreted in the Lab Reference for iPhone urine leading to nephrolithiasis (kidney stones) and sometimes renal failure. and iPod Touch Onset of PH2 is typically in childhood or adolescence with symptoms related to kidney stones. In some cases, kidney failure may be the initial presenting feature. Nephrocalcinosis, as seen by renal ultrasound, is observed less frequently in individuals with PH2 than primary Requires iOS 5.1+ hyperoxaluria type 1 (PH1). End-stage renal disease (ESRD) is also less common and of later onset than PH1; however, once ESRD develops, oxalate deposition in other organs such as REFERENCE VALUES bone, retina, and myocardium can occur. An interpretive report will be While the exact prevalence and incidence of PH2 are not known, it is thought that PH2 is less provided. common than PH1, which has an estimated prevalence rate of 1 to 3 per million population and an incidence of 0.1 per million/year. ANALYTIC TIME Biochemical testing is indicated in patients with possible primary hyperoxaluria. Measurement 14 days of urinary oxalate in a timed, 24-hour urine collection is strongly preferred, with correction to adult body surface area in pediatric patients (HYOX / Hyperoxaluria Panel, Urine; OXU / Oxalate, Urine). In very young children (incapable of performing a timed collection), random urine oxalate to creatinine ratios may be used for determination of oxalate excretion. -

Supplementary Materials
Supplementary Materials COMPARATIVE ANALYSIS OF THE TRANSCRIPTOME, PROTEOME AND miRNA PROFILE OF KUPFFER CELLS AND MONOCYTES Andrey Elchaninov1,3*, Anastasiya Lokhonina1,3, Maria Nikitina2, Polina Vishnyakova1,3, Andrey Makarov1, Irina Arutyunyan1, Anastasiya Poltavets1, Evgeniya Kananykhina2, Sergey Kovalchuk4, Evgeny Karpulevich5,6, Galina Bolshakova2, Gennady Sukhikh1, Timur Fatkhudinov2,3 1 Laboratory of Regenerative Medicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology Named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, Moscow, Russia 2 Laboratory of Growth and Development, Scientific Research Institute of Human Morphology, Moscow, Russia 3 Histology Department, Medical Institute, Peoples' Friendship University of Russia, Moscow, Russia 4 Laboratory of Bioinformatic methods for Combinatorial Chemistry and Biology, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia 5 Information Systems Department, Ivannikov Institute for System Programming of the Russian Academy of Sciences, Moscow, Russia 6 Genome Engineering Laboratory, Moscow Institute of Physics and Technology, Dolgoprudny, Moscow Region, Russia Figure S1. Flow cytometry analysis of unsorted blood sample. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S2. Flow cytometry analysis of unsorted liver stromal cells. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S3. MiRNAs expression analysis in monocytes and Kupffer cells. Full-length of heatmaps are presented. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Supplementary Methods
Supplementary methods Human lung tissues and tissue microarray (TMA) All human tissues were obtained from the Lung Cancer Specialized Program of Research Excellence (SPORE) Tissue Bank at the M.D. Anderson Cancer Center (Houston, TX). A collection of 26 lung adenocarcinomas and 24 non-tumoral paired tissues were snap-frozen and preserved in liquid nitrogen for total RNA extraction. For each tissue sample, the percentage of malignant tissue was calculated and the cellular composition of specimens was determined by histological examination (I.I.W.) following Hematoxylin-Eosin (H&E) staining. All malignant samples retained contained more than 50% tumor cells. Specimens resected from NSCLC stages I-IV patients who had no prior chemotherapy or radiotherapy were used for TMA analysis by immunohistochemistry. Patients who had smoked at least 100 cigarettes in their lifetime were defined as smokers. Samples were fixed in formalin, embedded in paraffin, stained with H&E, and reviewed by an experienced pathologist (I.I.W.). The 413 tissue specimens collected from 283 patients included 62 normal bronchial epithelia, 61 bronchial hyperplasias (Hyp), 15 squamous metaplasias (SqM), 9 squamous dysplasias (Dys), 26 carcinomas in situ (CIS), as well as 98 squamous cell carcinomas (SCC) and 141 adenocarcinomas. Normal bronchial epithelia, hyperplasia, squamous metaplasia, dysplasia, CIS, and SCC were considered to represent different steps in the development of SCCs. All tumors and lesions were classified according to the World Health Organization (WHO) 2004 criteria. The TMAs were prepared with a manual tissue arrayer (Advanced Tissue Arrayer ATA100, Chemicon International, Temecula, CA) using 1-mm-diameter cores in triplicate for tumors and 1.5 to 2-mm cores for normal epithelial and premalignant lesions. -

Severe Child Form of Primary Hyperoxaluria Type 2
Konkoľová et al. BMC Medical Genetics (2017) 18:59 DOI 10.1186/s12881-017-0421-8 CASE REPORT Open Access Severe child form of primary hyperoxaluria type 2 - a case report revealing consequence of GRHPR deficiency on metabolism Jana Konkoľová1,2*, Ján Chandoga1,2, Juraj Kováčik3, Marcel Repiský2, Veronika Kramarová2, Ivana Paučinová3 and Daniel Böhmer1,2 Abstract Background: Primary hyperoxaluria type 2 is a rare monogenic disorder inherited in an autosomal recessive pattern. It results from the absence of the enzyme glyoxylate reductase/hydroxypyruvate reductase (GRHPR). As a consequence of deficient enzyme activity, excessive amounts of oxalate and L-glycerate are excreted in the urine, and are a source for the formation of calcium oxalate stones that result in recurrent nephrolithiasis and less frequently nephrocalcinosis. Case presentation: We report a case of a 10-month-old patient diagnosed with urolithiasis. Screening of inborn errors of metabolism, including the performance of GC/MS urine organic acid profiling and HPLC amino acid profiling, showed abnormalities, which suggested deficiency of GRHPR enzyme. Additional metabolic disturbances observed in the patient led us to seek other genetic determinants and the elucidation of these findings. Besides the elevated excretion of 3-OH-butyrate, adipic acid, which are typical marks of ketosis, other metabolites such as 3- aminoisobutyric acid, 3-hydroxyisobutyric acid, 3-hydroxypropionic acid and 2-ethyl-3-hydroxypropionic acids were observed in increased amounts in the urine. Direct sequencing of the GRHPR gene revealed novel mutation, described for the first time in this article c.454dup (p.Thr152Asnfs*39) in homozygous form. The frequent nucleotide variants were found in AGXT2 gene. -

Abstracts 2019 Focus Sessions
52nd ANNUAL CONFERENCE Beyond Possible: Remarkable Transformation of Reproductive Biology Abstracts 2019 Focus Sessions S1.1 - The maternal lactocrine continuum programming uterine capacity. Frank Bartol, Jeffrey Vallet, Carol Bagnell Lactocrine signaling describes a mechanism by which milk-borne bioactive factors (MbFs) are communicated from mother to offspring by consequence of nursing. Lactocrine communication is an element of the maternal environmental continuum of factors that define pre- and postnatal developmental conditions and, therefore, the trajectory of mammalian development and offspring phenotype. Lactocrine-active factors can include MbFs of maternal origin, as well as factors of environmental origin to which the lactating female is exposed. Ideally, lactocrine signals communicated to nursing offspring insure a smooth transition and adaptation to extrauterine life. Disruption of lactocrine communication occurs when the quality or quantity of colostrum (first milk) is compromised. This can have lasting consequences for the health and fitness of nursing young. In the pig (Sus domesticus), imposition of a lactocrine-null state from birth (postnatal day = PND 0) by feeding milk replacer altered global patterns of uterine gene expression by PND 2, and inhibited endometrial development by PND 14. Similarly, lactocrine deficiency, reflecting minimal colostrum consumption from birth, retarded endometrial development in neonates, altered endometrial gene expression on pregnancy day (PxD) 13, and reduced live litter size in adult, neonatally lactocrine-deficient gilts. Elements of the lactocrine-sensitive, adult endometrial transcriptome at PxD 13 included factors affecting conceptus-endometrial interactions. A window for lactocrine programming of porcine endometrial function and uterine capacity was defined during the first 24 h of postnatal life. Lactocrine effects on the neonatal uterine miRNA-mRNA interactome at PND 2 implicated miRNAs as elements of the maternal lactocrine programming mechanism. -

Induced Interactions of Female Mouse Hypothalamic Proteins with Progestin Receptor-A in the Absence of Hormone
Received: 22 April 2020 | Revised: 24 August 2020 | Accepted: 25 August 2020 DOI: 10.1111/jne.12904 ORIGINAL ARTICLE Dopamine-induced interactions of female mouse hypothalamic proteins with progestin receptor-A in the absence of hormone Kalpana D. Acharya1 | Sabin A. Nettles1 | Cheryl F. Lichti2 | Katherine Warre-Cornish3,4 | Lucia Dutan Polit3,4 | Deepak P. Srivastava3,4 | Larry Denner5 | Marc J. Tetel1 1Neuroscience Department, Wellesley College, Wellesley, MA, USA Abstract 2Department of Pathology and Immunology, Neural progestin receptors (PR) function in reproduction, neural development, neu- Washington University School of Medicine, roprotection, learning, memory and the anxiety response. In the absence of pro- St Louis, MO, USA 3Department of Basic and Clinical gestins, PR can be activated by dopamine (DA) in the rodent hypothalamus to elicit Neuroscience, The Maurice Wohl Clinical female sexual behaviour. The present study investigated mechanisms of DA activa- Neuroscience Institute, Institute of Psychiatry Psychology and Neuroscience, tion of PR by testing the hypothesis that proteins from DA-treated hypothalami in- King's College London, London, UK teract with PR in the absence of progestins. Ovariectomised, oestradiol-primed mice 4 MRC Centre for Neurodevelopmental were infused with a D1-receptor agonist, SKF38393 (SKF), into the third ventricle Disorders, King’s College London, London, UK 30 minutes prior to death. Proteins from SKF-treated hypothalami were pulled-down 5Department of Internal Medicine, with glutathione S-transferase-tagged mouse PR-A or PR-B and the interactomes University of Texas Medical Branch, were analysed by mass spectrometry. The largest functional group to interact with Galveston, TX, USA PR-A in a DA-dependent manner was synaptic proteins. -

Mannose-Containing Solution for Lyophilization
(19) TZZ _Z_ZZ_T (11) EP 2 510 100 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/87 (2006.01) C12N 15/11 (2006.01) 11.10.2017 Bulletin 2017/41 (86) International application number: (21) Application number: 10779700.3 PCT/EP2010/006788 (22) Date of filing: 08.11.2010 (87) International publication number: WO 2011/069586 (16.06.2011 Gazette 2011/24) (54) MANNOSE-CONTAINING SOLUTION FOR LYOPHILIZATION, TRANSFECTION AND/OR INJECTION OF NUCLEIC ACIDS MANNOSE-ENTHALTENDE LÖSUNG ZUR LYOPHILISIERUNG, TRANSFEKTION UND/ODER INJEKTION VON NUKLEINSÄUREN SOLUTION CONTENANT MANNOSE POUR LA LYOPHILISATION, TRANSFECTION ET/OU INJECTION D’ACIDES NUCLÉIQUES (84) Designated Contracting States: (56) References cited: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB EP-A1- 2 431 405 WO-A2-2009/125986 GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR • NAKAMURA K. ET AL.: "Enhanced gene transfection in macrophages by (30) Priority: 09.12.2009 PCT/EP2009/008804 Histidine-conjugated mannosylated cationic liposomes", BIOL. PHARM. BULL., vol. 32, no. 9, (43) Date of publication of application: September 2009 (2009-09), pages 1628-1631, 17.10.2012 Bulletin 2012/42 XP002581475, • POXON S W ET AL: "The effect of lyophilization (73) Proprietor: CureVac AG on plasmid DNA activity", PHARMACEUTICAL 72076 Tübingen (DE) DEVELOPMENT AND TECHNOLOGY, NEW YORK, NY, US, vol. 5, no. 1, 1 January 2000 (72) Inventor: MUTZKE, Thorsten (2000-01-01) , pages 115-122, XP009131956, 72760 Reutlingen (DE) ISSN: 1083-7450 cited in the application • JONES K.L., DRANE D. -

Structure-Based Targeting of Transcriptional Regulatory Complexes Implicated in Human Disease: a Dissertation
University of Massachusetts Medical School eScholarship@UMMS GSBS Dissertations and Theses Graduate School of Biomedical Sciences 2013-07-19 Structure-based Targeting of Transcriptional Regulatory Complexes Implicated in Human Disease: A Dissertation Brendan J. Hilbert University of Massachusetts Medical School Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/gsbs_diss Part of the Biochemistry Commons, Molecular Biology Commons, Molecular Genetics Commons, and the Structural Biology Commons Repository Citation Hilbert BJ. (2013). Structure-based Targeting of Transcriptional Regulatory Complexes Implicated in Human Disease: A Dissertation. GSBS Dissertations and Theses. https://doi.org/10.13028/M23K66. Retrieved from https://escholarship.umassmed.edu/gsbs_diss/681 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in GSBS Dissertations and Theses by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. STRUCTURE-BASED TARGETING OF TRANSCRIPTIONAL REGULATORY COMPLEXES IMPLICATED IN HUMAN DISEASE A Dissertation Presented By Brendan Jay Hilbert Submitted to the Faculty of the University of Massachusetts Graduate School of Biomedical Sciences, Worcester in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY July 19, 2013 Biochemistry and Molecular Pharmacology ii STRUCTURE-BASED TARGETING OF TRANSCRIPTIONAL REGULATORY COMPLEXES IMPLICATED IN HUMAN DISEASE A Dissertation Presented By Brendan Jay Hilbert The signatures of the Dissertation Defense Committee signify completion and approval as to the style and content of the Dissertation. Celia A. Schiffer, Ph.D., Thesis Advisor William Royer Jr., Ph. D., Thesis Advisor David G. Lambright, Ph.D., Member of Committee Brian A. -
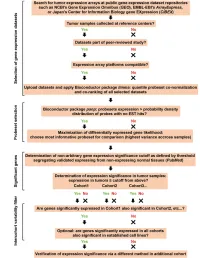
Supplementary Data
SUPPLEMENTAL INFORMATION A study restricted to chemokine receptors as well as a genome-wide transcript analysis uncovered CXCR4 as preferentially expressed in Ewing's sarcoma (Ewing's sarcoma) cells of metastatic origin (Figure 4). Transcriptome analyses showed that in addition to CXCR4, genes known to support cell motility and invasion topped the list of genes preferentially expressed in metastasis-derived cells (Figure 4D). These included kynurenine 3-monooxygenase (KMO), galectin-1 (LGALS1), gastrin-releasing peptide (GRP), procollagen C-endopeptidase enhancer (PCOLCE), and ephrin receptor B (EPHB3). KMO, a key enzyme of tryptophan catabolism, has not been linked to metastasis. Tryptophan and its catabolites, however, are involved in immune evasion by tumors, a process that can assist in tumor progression and metastasis (1). LGALS1, GRP, PCOLCE and EPHB3 have been linked to tumor progression and metastasis of several cancers (2-4). Top genes preferentially expressed in L-EDCL included genes that suppress cell motility and/or potentiate cell adhesion such as plakophilin 1 (PKP1), neuropeptide Y (NPY), or the metastasis suppressor TXNIP (5-7) (Figure 4D). Overall, L-EDCL were enriched in gene sets geared at optimizing nutrient transport and usage (Figure 4D; Supplementary Table 3), a state that may support the early stages of tumor growth. Once tumor growth outpaces nutrient and oxygen supplies, gene expression programs are usually switched to hypoxic response and neoangiogenesis, which ultimately lead to tumor egress and metastasis. Accordingly, gene sets involved in extracellular matrix remodeling, MAPK signaling, and response to hypoxia were up-regulated in M-EDCL (Figure 4D; Supplementary Table 4), consistent with their association to metastasis in other cancers (8, 9). -

Proteomic Analysis Identifies Distinct Glomerular Extracellular Matrix In
CLINICAL RESEARCH www.jasn.org Proteomic Analysis Identifies Distinct Glomerular Extracellular Matrix in Collapsing Focal Segmental Glomerulosclerosis Michael L. Merchant,1 Michelle T. Barati,1 Dawn J. Caster ,1 Jessica L. Hata,2 Liliane Hobeika,3 Susan Coventry,2 Michael E. Brier,1 Daniel W. Wilkey,1 Ming Li,1 Ilse M. Rood,4 Jeroen K. Deegens,4 Jack F. Wetzels,4 Christopher P. Larsen,5 Jonathan P. Troost,6 Jeffrey B. Hodgin,7 Laura H. Mariani,8 Matthias Kretzler ,8 Jon B. Klein,1,9 and Kenneth R. McLeish1 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background The mechanisms leading to extracellular matrix (ECM) replacement of areas of glomerular capillaries in histologic variants of FSGS are unknown. This study used proteomics to test the hypothesis that glomerular ECM composition in collapsing FSGS (cFSGS) differs from that of other variants. Methods ECM proteins in glomeruli from biopsy specimens of patients with FSGS not otherwise specified (FSGS-NOS) or cFSGS and from normal controls were distinguished and quantified using mass spectrom- etry, verified and localized using immunohistochemistry (IHC) and confocal microscopy, and assessed for gene expression. The analysis also quantified urinary excretion of ECM proteins and peptides. Results Of 58 ECM proteins that differed in abundance between cFSGS and FSGS-NOS, 41 were more abundant in cFSGS and 17 in FSGS-NOS. IHC showed that glomerular tuft staining for cathepsin B, ca- thepsin C, and annexin A3 in cFSGS was significantly greater than in other FSGS variants, in minimal change disease, or in membranous nephropathy.