Induced Interactions of Female Mouse Hypothalamic Proteins with Progestin Receptor-A in the Absence of Hormone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Evidence for Gliadin Antibodies As Causative Agents in Schizophrenia
1 Evidence for gliadin antibodies as causative agents in schizophrenia. C.J.Carter PolygenicPathways, 20 Upper Maze Hill, Saint-Leonard’s on Sea, East Sussex, TN37 0LG [email protected] Tel: 0044 (0)1424 422201 I have no fax Abstract Antibodies to gliadin, a component of gluten, have frequently been reported in schizophrenia patients, and in some cases remission has been noted following the instigation of a gluten free diet. Gliadin is a highly immunogenic protein, and B cell epitopes along its entire immunogenic length are homologous to the products of numerous proteins relevant to schizophrenia (p = 0.012 to 3e-25). These include members of the DISC1 interactome, of glutamate, dopamine and neuregulin signalling networks, and of pathways involved in plasticity, dendritic growth or myelination. Antibodies to gliadin are likely to cross react with these key proteins, as has already been observed with synapsin 1 and calreticulin. Gliadin may thus be a causative agent in schizophrenia, under certain genetic and immunological conditions, producing its effects via antibody mediated knockdown of multiple proteins relevant to the disease process. Because of such homology, an autoimmune response may be sustained by the human antigens that resemble gliadin itself, a scenario supported by many reports of immune activation both in the brain and in lymphocytes in schizophrenia. Gluten free diets and removal of such antibodies may be of therapeutic benefit in certain cases of schizophrenia. 2 Introduction A number of studies from China, Norway, and the USA have reported the presence of gliadin antibodies in schizophrenia 1-5. Gliadin is a component of gluten, intolerance to which is implicated in coeliac disease 6. -

Concentrative Nucleoside Transporter 3 As a Prognostic Indicator for Favorable Outcome of T(8;21)-Positive Acute Myeloid Leukemi
488 ONCOLOGY REPORTS 34: 488-494, 2015 Concentrative nucleoside transporter 3 as a prognostic indicator for favorable outcome of t(8;21)-positive acute myeloid leukemia patients after cytarabine-based chemotherapy JU HAN SONG1, KYUNG-MIN CHO1, HYEOUNG-JOON KIM2, YEO-KYEOUNG KIM2, NAN YOUNG KIM2, HEE-JE KIM3, TAE-Hyang LEE3, SEUNG YONG Hwang4 and TAE SUNG KIM1 1Division of Life Sciences, School of Life Sciences and Biotechnology, Korea University, Seoul; 2Genome Research Center for Hematopoietic Diseases, Chonnam National University Hwasun Hospital, Hwasun; 3Catholic Blood and Marrow Transplantation Center, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul; 4Division of Molecular and Life Science and GenoCheck Co., Ltd., Hanyang University, Ansan, Republic of Korea Received January 20, 2015; Accepted March 18, 2015 DOI: 10.3892/or.2015.3959 Abstract. Although acute myeloid leukemia (AML) exhibits Introduction diverse responses to chemotherapy, patients harboring the t(8;21) translocation are part of a favorable risk group. Acute myeloid leukemia (AML) is a highly heterogeneous However, the reason why this subgroup is more responsive hematologic malignancy that displays diverse responses to cytarabine-based therapy has not been elucidated. In the to chemotherapy. Although a number of clinical factors present study, we analyzed expression levels of cytarabine affect treatment outcomes, the cytogenetic features of AML metabolism-related genes in patients diagnosed with AML are generally accepted as strong predictors of therapeutic with or without t(8;21) and investigated their correlation with response (1). Pediatric and adult patients carrying the t(8;21) clinical outcomes after cytarabine-based therapy. Among the chromosomal translocation, which is one of the most frequent 8 genes studied, expression of the concentrative nucleoside AML subtypes, are part of a favorable risk group (2). -

Mammalian 5€²-Nucleotidases*
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 278, No. 47, Issue of November 21, pp. 46195–46198, 2003 Minireview © 2003 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. -Mammalian 5-Nucleotidases* residues the best alignment was between the two deoxynucle otidases and cN-III (10). Two 5Ј-nucleotidases, cN-II and cN- III, exhibit phosphotransferase activity (for reviews see Refs. Published, JBC Papers in Press, August 28, 2003, DOI 10.1074/jbc.R300032200 14 and 15) possibly because of higher stability of the phos- phoenzyme intermediate or faster exchange of the nucleoside Vera Bianchi‡§ and Jozef Spychala¶ product with the nucleoside acceptor. From the ‡Department of Biology, University of Padova, The active site of E. coli 5Ј-nucleotidase, the paradigm for I-35131 Padova, Italy and the ¶Lineberger eN, contains two zinc ions and the catalytic dyad Asp-His (11). Comprehensive Cancer Center, University of North No phosphoenzyme intermediate is formed during catalysis, Carolina, Chapel Hill, North Carolina 27599-7295 but a water molecule performs the nucleophilic attack on the phosphate (16). Nucleoside monophosphate phosphohydrolases or 5Ј-nucle- otidases (members of EC 3.1.3.5 and EC 3.1.3.6) dephosphoryl- Properties, Detection, and Inhibition ate non-cyclic nucleoside monophosphates to nucleosides and of 5-Nucleotidases inorganic phosphate. Seven human 5Ј-nucleotidases with dif- All 5Ј-nucleotidases have relatively broad substrate specific- ferent subcellular localization have been cloned (Table I). Se- ities. In agreement with the structural information on the quence comparisons show high homology only between cytoso- active sites (10, 11), all family members except eN are abso- lic 5Ј-nucleotidase IA (cN-IA)1 and B and between cytosolic lutely dependent on magnesium for activity. -

Differential Physiological Role of BIN1 Isoforms in Skeletal Muscle Development, Function and Regeneration
bioRxiv preprint doi: https://doi.org/10.1101/477950; this version posted December 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Differential physiological role of BIN1 isoforms in skeletal muscle development, function and regeneration Ivana Prokic1,2,3,4, Belinda Cowling1,2,3,4, Candice Kutchukian5, Christine Kretz1,2,3,4, Hichem Tasfaout1,2,3,4, Josiane Hergueux1,2,3,4, Olivia Wendling1,2,3,4, Arnaud Ferry10, Anne Toussaint1,2,3,4, Christos Gavriilidis1,2,3,4, Vasugi Nattarayan1,2,3,4, Catherine Koch1,2,3,4, Jeanne Lainné6,7, Roy Combe2,3,4,8, Laurent Tiret9, Vincent Jacquemond5, Fanny Pilot-Storck9, Jocelyn Laporte1,2,3,4 1Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), Illkirch, France 2Centre National de la Recherche Scientifique (CNRS), UMR7104, Illkirch, France 3Institut National de la Santé et de la Recherche Médicale (INSERM), U1258, Illkirch, France 4Université de Strasbourg, Illkirch, France 5Univ Lyon, Université Claude Bernard Lyon 1, CNRS UMR-5310, INSERM U-1217, Institut NeuroMyoGène, 8 avenue Rockefeller, 69373 Lyon, France 6Sorbonne Université, INSERM, Institute of Myology, Centre of Research in Myology, UMRS 974, F- 75013, Paris, France 7Sorbonne Université, Department of Physiology, UPMC Univ Paris 06, Pitié-Salpêtrière Hospital, F- 75013, Paris, France 8CELPHEDIA-PHENOMIN, Institut Clinique de la Souris (ICS), Illkirch, France 9U955 – IMRB, Team 10 - Biology of the neuromuscular system, Inserm, UPEC, Ecole nationale vétérinaire d’Alfort, Maisons-Alfort, 94700, France 10Sorbonne Université, INSERM, Institute of Myology, Centre of Research in Myology, UMRS 794, F- 75013, Paris, France Correspondence to: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/477950; this version posted December 11, 2018. -
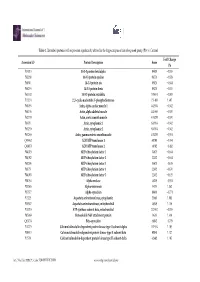
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

DISSERTATION PROTEOMIC PROFILING of the RAT RENAL PROXIMAL CONVOLUTED TUBULE in RESPONSE to CHRONIC METABOLIC ACIDOSIS Submitted
DISSERTATION PROTEOMIC PROFILING OF THE RAT RENAL PROXIMAL CONVOLUTED TUBULE IN RESPONSE TO CHRONIC METABOLIC ACIDOSIS Submitted by Dana Marie Freund Department of Biochemistry and Molecular Biology In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Spring 2013 Doctoral Committee: Advisor: Norman Curthoys Co-Advisor: Jessica Prenni Jennifer Nyborg Olve Peersen Karen Dobos ABSTRACT PROTEOMIC PROFILING OF THE RAT RENAL PROXIMAL CONVOLUTED TUBULE IN RESPONSE TO CHRONIC METABOLIC ACIDOSIS The human kidneys contain more than one million glomeruli which filter nearly 200 liters of plasma per day. The proximal tubule is the segment of the nephron that immediately follows the glomeruli. This portion of the nephron contributes to fluid, electrolyte and nutrient homeostasis by reabsorbing 60-70% of the filtered water and NaCl and an even greater proportion of NaHCO3. The initial or convoluted portion of the proximal tubule reabsorbs nearly all of the nutrients in the glomerular filtrate and is the site of active secretion and many of the metabolic functions of the kidney. For example, the proximal convoluted tubule is the primary site of renal ammoniagenesis and gluconeogenesis, processes that are significantly activated during metabolic acidosis. Metabolic acidosis is a common clinical condition that is characterized by a decrease in blood pH and bicarbonate concentration. Metabolic acidosis also occurs frequently as a secondary complication, which adversely affects the outcome of patients with various life- threatening conditions. This type of acidosis can occur acutely, lasting for a few hours to a day, or as a chronic condition where acid-base balance is not fully restored. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

1 Metabolic Dysfunction Is Restricted to the Sciatic Nerve in Experimental
Page 1 of 255 Diabetes Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy Oliver J. Freeman1,2, Richard D. Unwin2,3, Andrew W. Dowsey2,3, Paul Begley2,3, Sumia Ali1, Katherine A. Hollywood2,3, Nitin Rustogi2,3, Rasmus S. Petersen1, Warwick B. Dunn2,3†, Garth J.S. Cooper2,3,4,5* & Natalie J. Gardiner1* 1 Faculty of Life Sciences, University of Manchester, UK 2 Centre for Advanced Discovery and Experimental Therapeutics (CADET), Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK 3 Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, UK 4 School of Biological Sciences, University of Auckland, New Zealand 5 Department of Pharmacology, Medical Sciences Division, University of Oxford, UK † Present address: School of Biosciences, University of Birmingham, UK *Joint corresponding authors: Natalie J. Gardiner and Garth J.S. Cooper Email: [email protected]; [email protected] Address: University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, United Kingdom Telephone: +44 161 275 5768; +44 161 701 0240 Word count: 4,490 Number of tables: 1, Number of figures: 6 Running title: Metabolic dysfunction in diabetic neuropathy 1 Diabetes Publish Ahead of Print, published online October 15, 2015 Diabetes Page 2 of 255 Abstract High glucose levels in the peripheral nervous system (PNS) have been implicated in the pathogenesis of diabetic neuropathy (DN). However our understanding of the molecular mechanisms which cause the marked distal pathology is incomplete. Here we performed a comprehensive, system-wide analysis of the PNS of a rodent model of DN. -

Mechanisms of Α-Synuclein Induced Synaptopathy in Parkinson’S Disease
King’s Research Portal DOI: 10.3389/fnins.2018.00080 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Bridi, J. C., & Hirth, F. (2018). Mechanisms of -Synuclein Induced Synaptopathy in Parkinson's Disease. Frontiers in Neuroscience, 12, 80. DOI: 10.3389/fnins.2018.00080 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Grhpr Gene, Full Gene Analysis
TEST ID: GRHPZ GRHPR GENE, FULL GENE ANALYSIS CLINICAL INFORMATION MOBILE APPS FROM MAYO MEDICAL LABORATORIES Primary hyperoxaluria type 2 (PH2) is a hereditary disorder of glyoxylate metabolism caused by deficiency of the hepatic enzyme glyoxylate reductase/hydroxypyruvate reductase (GRHPR). Lab Catalog for iPad and Absence of GRHPR activity results in excess oxalate and usually L-glycerate excreted in the Lab Reference for iPhone urine leading to nephrolithiasis (kidney stones) and sometimes renal failure. and iPod Touch Onset of PH2 is typically in childhood or adolescence with symptoms related to kidney stones. In some cases, kidney failure may be the initial presenting feature. Nephrocalcinosis, as seen by renal ultrasound, is observed less frequently in individuals with PH2 than primary Requires iOS 5.1+ hyperoxaluria type 1 (PH1). End-stage renal disease (ESRD) is also less common and of later onset than PH1; however, once ESRD develops, oxalate deposition in other organs such as REFERENCE VALUES bone, retina, and myocardium can occur. An interpretive report will be While the exact prevalence and incidence of PH2 are not known, it is thought that PH2 is less provided. common than PH1, which has an estimated prevalence rate of 1 to 3 per million population and an incidence of 0.1 per million/year. ANALYTIC TIME Biochemical testing is indicated in patients with possible primary hyperoxaluria. Measurement 14 days of urinary oxalate in a timed, 24-hour urine collection is strongly preferred, with correction to adult body surface area in pediatric patients (HYOX / Hyperoxaluria Panel, Urine; OXU / Oxalate, Urine). In very young children (incapable of performing a timed collection), random urine oxalate to creatinine ratios may be used for determination of oxalate excretion. -

The Emerging Spectrum of Allelic Variation in Schizophrenia
Molecular Psychiatry (2013) 18, 38 -- 52 & 2013 Macmillan Publishers Limited All rights reserved 1359-4184/13 www.nature.com/mp EXPERT REVIEW The emerging spectrum of allelic variation in schizophrenia: current evidence and strategies for the identification and functional characterization of common and rare variants BJ Mowry1,2 and J Gratten1 After decades of halting progress, recent large genome-wide association studies (GWAS) are finally shining light on the genetic architecture of schizophrenia. The picture emerging is one of sobering complexity, involving large numbers of risk alleles across the entire allelic spectrum. The aims of this article are to summarize the key genetic findings to date and to compare and contrast methods for identifying additional risk alleles, including GWAS, targeted genotyping and sequencing. A further aim is to consider the challenges and opportunities involved in determining the functional basis of genetic associations, for instance using functional genomics, cellular models, animal models and imaging genetics. We conclude that diverse approaches will be required to identify and functionally characterize the full spectrum of risk variants for schizophrenia. These efforts should adhere to the stringent standards of statistical association developed for GWAS and are likely to entail very large sample sizes. Nonetheless, now more than any previous time, there are reasons for optimism and the ultimate goal of personalized interventions and therapeutics, although still distant, no longer seems unattainable. Molecular Psychiatry (2013) 18, 38--52; doi:10.1038/mp.2012.34; published online 1 May 2012 Keywords: CNV; functional genomics; GWAS; schizophrenia; sequencing; SNP THE NATURE OF THE PROBLEM complications (obesity, nicotine dependence, metabolic syndrome 13 Schizophrenia is a chronic psychiatric disorder characterized by and premature mortality), low employment and substantial 14 delusional beliefs, auditory hallucinations, disorganized thought homelessness. -

Murine Megakaryopoiesis Is Critical for P21 SCL-Mediated Regulation Of
From bloodjournal.hematologylibrary.org at UNIVERSITY OF BIRMINGHAM on March 1, 2012. For personal use only. 2011 118: 723-735 Prepublished online May 19, 2011; doi:10.1182/blood-2011-01-328765 SCL-mediated regulation of the cell-cycle regulator p21 is critical for murine megakaryopoiesis Hedia Chagraoui, Mira Kassouf, Sreemoti Banerjee, Nicolas Goardon, Kevin Clark, Ann Atzberger, Andrew C. Pearce, Radek C. Skoda, David J. P. Ferguson, Steve P. Watson, Paresh Vyas and Catherine Porcher Updated information and services can be found at: http://bloodjournal.hematologylibrary.org/content/118/3/723.full.html Articles on similar topics can be found in the following Blood collections Platelets and Thrombopoiesis (260 articles) Information about reproducing this article in parts or in its entirety may be found online at: http://bloodjournal.hematologylibrary.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://bloodjournal.hematologylibrary.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://bloodjournal.hematologylibrary.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved. From bloodjournal.hematologylibrary.org at UNIVERSITY OF BIRMINGHAM on March 1, 2012. For personal use only. PLATELETS AND THROMBOPOIESIS SCL-mediated regulation of the cell-cycle regulator p21 is critical for murine megakaryopoiesis Hedia Chagraoui,1 *Mira Kassouf,1 *Sreemoti Banerjee,1 Nicolas Goardon,1 Kevin Clark,1 Ann Atzberger,1 Andrew C.