Phylogenetic Analysis of Freshwater Crayfish of Massachusetts: the Genus Procambarus ______
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decapoda: Cambaridae) of Arkansas Henry W
Journal of the Arkansas Academy of Science Volume 71 Article 9 2017 An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas Henry W. Robison Retired, [email protected] Keith A. Crandall George Washington University, [email protected] Chris T. McAllister Eastern Oklahoma State College, [email protected] Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Robison, Henry W.; Crandall, Keith A.; and McAllister, Chris T. (2017) "An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas," Journal of the Arkansas Academy of Science: Vol. 71 , Article 9. Available at: http://scholarworks.uark.edu/jaas/vol71/iss1/9 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. An Annotated Checklist of the Crayfishes (Decapoda: Cambaridae) of Arkansas Cover Page Footnote Our deepest thanks go to HWR’s numerous former SAU students who traveled with him in search of crayfishes on many fieldtrips throughout Arkansas from 1971 to 2008. Personnel especially integral to this study were C. -

Species Assessment for Atlantic Coast Leopard Frog
Species Status Assessment Class: Amphibia Family: Ranidae Scientific Name: Lithobates [Rana] kauffeldi Common Name: Atlantic Coast leopard frog Species synopsis: More than a century of taxonomic confusion regarding the leopard frogs of the East Coast was resolved in 2012 with the publication of a genetic analysis (Newman et al. 2012) confirming that a third, cryptic species of leopard frog (Rana [= Lithobates] sp. nov.) occurs in southern New York, northern New Jersey, and western Connecticut. The molecular evidence strongly supported the distinction of this new species from the previously known northern (R. pipiens [= L. pipiens]) and southern (R. sphenocephala [=L. sphenocephalus]) leopard frogs. Rana kauffeldi is morphologically similar to R. sphenocephala and R. pipiens, but distinguishable by advertisement call, genetics, habitat, geographic distribution, and a combination of morphological characters (Feinberg et al. 2014). Bioacoustic evidence of the frog’s occurrence in southern New Jersey, Maryland, Delaware, and as far south as the Virginia/North Carolina border is available, thereby raising uncertainty about which species of leopard frog occur(s) presently and historically throughout the region. Some evidence suggests that Long Island might at one time have had two species: the southern leopard frog in the pine barrens and the Atlantic Coast leopard frog in coastal wetlands and the Hudson Valley. For simplicity’s sake, in this assessment we retain the name “Atlantic Coast leopard frog” even though much of the information available may also refer to the southern leopard frog or a combination of species (Feinberg et al. 2014). 1 I. Status a. Current and Legal Protected Status i. Federal ____ Not Listed______________________ Candidate? ___No____ ii. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Brazos Dwarf Crayfish (Cambarellus Texanus) Ecological Risk Screening Summary
Brazos Dwarf Crayfish (Cambarellus texanus) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, April 2014 Revised, October 2016 Web Version, 11/17/2017 Photo: © Keith A. Crandall. Licensed under Creative Commons Attribution-NonCommercial- ShareAlike License. Available: http://tolweb.org/Cambarellus_(Pandicambarus)_texanus. (October 2016). 1 Native Range and Status in the United States Native Range From Fetzner (2016): “East of the Lavaca River and Bay to the Brazos River drainage system, Texas.” Status in the United States From Alvarez et al. (2010): “This species was first found in a ditch near Bay City in Matagorda County, Texas. It has since been found in the Colorado River, Fort Bend County, and Waller County. It is thought that the range is bound by the Lavaca River and Bay on the west, though the northward and eastward range limits are not known (Albaugh and Black 1973).” “This species has been collected from 31 sites and is believed to be common at most sites (D. Johnson pers. comm. 2009).” 1 Means of Introductions in the United States This species has not been reported as introduced outside of its native range in the United States. Remarks From NatureServe (2015): “It is found only in Texas in a small range near the central Texas coast (Johnson and Johnson, 2008). It has a larger range than Cambarellus ninae, but does occur in an area that is experiencing urban growth; however populations appear stable and there is no evidence of decline.” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS -

Behaviorální Odpověď Čolka Obecného (Lissotriton Vulgaris) V Konfrontaci S Původními a Nepůvodními Predátory
Univerzita Palackého v Olomouci Přírodovědecká fakulta Katedra ekologie a životního prostředí Behaviorální odpověď čolka obecného (Lissotriton vulgaris) v konfrontaci s původními a nepůvodními predátory Marcela Janků Bakalářská práce předložená na Katedře ekologie a životního prostředí Přírodovědecké fakulty Univerzity Palackého v Olomouci jako součást požadavků na získání titulu Bc. v oboru Ekologie a ochrana životního prostředí Vedoucí práce: Mgr. Vendula Kurdíková Olomouc 2017 © Marcela Janků, 2017 Janků, M. 2017. Behaviorální odpověď čolka obecného (Lissotriton vulgaris) v konfrontaci s původními a nepůvodními predátory [bakalářská práce]. Olomouc: Katedra ekologie a ŽP PřF UP v Olomouci. 46 s. Česky. Abstrakt Mnoho živočichů včetně obojživelníků je schopno měnit své chování na základě přítomnosti predátora, kterého detekují podle vizuálních či chemických signálů. Řada recentních výzkumů ukazuje, že behaviorální odpověď se liší podle druhu predátora a podmínek prostředí. Cílem této práce je experimentálně zhodnotit reakci čolka obecného (Lissotriton vulgaris) na dva původní druhy (karas stříbřitý – Carassius gibelio a plotice obecná – Rutilus rutilus) a dva nepůvodní druhy (rak signální – Pacifastacus leniusculus a střevlička východní – Pseudorasbora parva) České republiky a zjistit rozdíl reakcí čolka na tyto predátory. Byly provedeny dva typy pokusů, olfaktoricko-vizuální typ kontaktu a vizuální kontakt. Dále jsme porovnali behaviorální odpověď samců a samic čolka obecného. Zkoumaní jedinci vykazovali prokazatelné snížení konzumace potravy v přítomnosti predátora oproti kontrole. Byl zaznamenám signifikantní rozdíl v konzumaci potravy během kontaktu s původními a nepůvodními predátory. V případě nepůvodních druhů konzumovali čolci méně potravy než v přítomnosti původních druhů. Olfaktoricko-vizuální typ detekce byl pro jedince efektivnější, čolci při něm snižovali svou potravní aktivitu oproti vizuálnímu kontaktu. U samic byl příjem potravy nižší než u samců. -

First Records of Alien Crayfish of the Procambarus Acutus Species Complex in Belgium
BioInvasions Records (2020) Volume 9, Issue 3: 562–569 CORRECTED PROOF Rapid Communication First records of alien crayfish of the Procambarus acutus species complex in Belgium Kevin Scheers1,2,*,+, Pieter Boets3,4,+, Thomas Abeel5 and Tom Van den Neucker2,6 1Research Institute for Nature and Forest (INBO), Havenlaan 88 bus 73, 1000 Brussels, Belgium 2Biodiversity Inventory for Conservation NPO (BINCO), Walmersumstraat 44, 3380 Glabbeek, Belgium 3Provincial Centre of Environmental Research, Godshuizenlaan 95, 9000 Ghent, Belgium 4Ghent University, Department of Animal Science and Aquatic Ecology, Coupure Links 653, 9000 Ghent, Belgium 5Odisee Campus Sint-Niklaas, Aqua-ERF, Hospitaalstraat 23, 9100 Sint-Niklaas, Belgium 6University of Antwerp, Department of Biology, Ecosystem Management Research Group, Universiteitsplein 1C, 2160 Wilrijk, Belgium + both authors contributed equally Author e-mails: [email protected] (KS), [email protected] (PB), [email protected] (TA) , [email protected] (TVDN) *Corresponding author Citation: Scheers K, Boets P, Abeel T, Van den Neucker T (2020) First records of Abstract alien crayfish of the Procambarus acutus species complex in Belgium. BioInvasions We present the first Belgian records of potentially invasive alien crayfish of the Records 9(3): 562–569, https://doi.org/10. Procambarus acutus species complex, including the first confirmed record of P. acutus 3391/bir.2020.9.3.11 acutus. The species complex was observed at four different sites in three provinces Received: 5 February 2020 in the north of the country. Only at one site the presence of a form I male specimen Accepted: 14 May 2020 made identification to species level possible, based on gonopod morphology. -

Southern Leopard Frog
Species Status Assessment Class: Amphibia Family: Ranidae Scientific Name: Lithobates sphenocephalus utricularius Common Name: Southern leopard frog Species synopsis: NOTE: More than a century of taxonomic confusion regarding the leopard frogs of the East Coast was resolved in 2012 with the publication of a genetic analysis (Newman et al. 2012) confirming that a third, cryptic species of leopard frog (Rana [= Lithobates] sp. nov.) occurs in southern New York, northern New Jersey, and western Connecticut. The molecular evidence strongly supported the distinction of this new species from the previously known northern (R. pipiens [= L. pipiens]) and southern (R. sphenocephala [=L. sphenocephalus]) leopard frogs. The new species’ formal description, which presents differences in vocalizations, morphology, and habitat affiliation (Feinberg et al. in preparation), is nearing submission for publication. This manuscript also presents bioacoustic evidence of the frog’s occurrence in southern New Jersey, Maryland, Delaware, and as far south as the Virginia/North Carolina border, thereby raising uncertainty about which species of leopard frog occur(s) presently and historically throughout the region. Some evidence suggests that Long Island might at one time have had two species: the southern leopard frog in the pine barrens and the undescribed species in coastal wetlands and the Hudson Valley. For simplicity’s sake, in this assessment we retain the name “southern leopard frog” even though much of the information available may refer to the undescribed species or a combination of species. The southern leopard frog occurs in the eastern United States and reaches the northern extent of its range in the lower Hudson Valley of New York. -
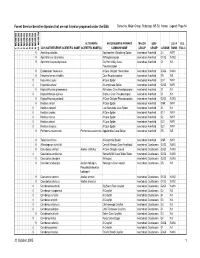
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Implications for the Evolutionary Ecology of Crayfish (Decapoda: Cambaridae)
SPECIES DISTRIBUTIONS AND TRAIT- ENVIRONMENT CORRELATIONS: IMPLICATIONS FOR THE EVOLUTIONARY ECOLOGY OF CRAYFISH (DECAPODA: CAMBARIDAE) By REID LANDEN MOREHOUSE Bachelor of Science in Fisheries and Aquatic Sciences Purdue University West Lafayette, IN 2006 Master of Science in Zoology Oklahoma State University Stillwater, OK 2010 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY July, 2014 SPECIES DISTRIBUTIONS AND TRAIT- ENVIRONMENT CORRELATIONS: IMPLICATIONS FOR THE EVOLUTIONARY ECOLOGY OF CRAYFISH (DECAPODA: CAMBARIDAE) Dissertation Approved: Dr. Michael Tobler Dissertation Adviser Dr. Punidan Jeyasingh Dr. Monica Papeş Dr. Andrew Dzialowski Dr. Shannon Brewer ii ACKNOWLEDGEMENTS This research would not have been possible without the advice, guidance, counseling and help from my friends and family. First, I am in great debt to my advisor, Dr. Michi Tobler. Without your never ending help and desire to push me to my limits, these projects would not have been possible. Your attitude towards me and my research allowed me to really dive right in without feeling pressured and stressed. The freedom and trust you gave me to run my own research allowed me to grow as an individual more than I ever would have thought. I could not have asked for a better advisor and friend. Next, I thank my committee members: Dr. Puni Jeyasingh, Dr. Andrew Dzialowski, Dr. Mona Papeş, and Dr. Shannon Brewer, for all of your faith and confidence in my research and myself. Your jokes, even at my expense, and overall general attitudes really help me keep a level head and push through the rough times. -

White River Crayfish Procambarusprocambarus Acutus Aacutushemimysis Acutus Acutus Anomala
White River Crayfish www.seagrant.psu.edu ProcambarusProcambarus acutus aacutusHemimysis acutus acutus anomala The white river crayfish, also called the white river crawfish and the eastern white river crayfish, is often confused with its southern counterpart, the southern white river crayfish (Procambarus zonangulus). The eastern white river crayfish occurs naturally in the United States and is cultured eastwards from Lousiana to the Atlantic coast northward to Maine, Photo courtesy of Tony Palacios, but has established select non-native populations in locations throughout the East Coast iNaturalist.org, EOL. and in California. Species Description Adult white river crayfish are usually a dark burgundy red but can range in color from pinkish tan to brownish olive with a black “V-shaped” stripe on the abdomen. The carapace is rough and granular and is separated in the middle by a narrow space called the areola. Juveniles are gray with dark spots scattered over the carapace. The claws are long and narrow, delicate in appearance, and have small dark tubercles. This species reaches about 6-13 cm (2.5-5 in) in length. The white river crayfish is nearly impossible to distinguish from the southern white river crayfish without looking at the reproductive structures of a breeding male. It is also confused with the red swamp crayfish (P. clarkii), which have an areola that is straight, or often invisible, and a black “V-shaped” stripe on the abdomen. The juveniles are also typically plain or striped on the carapace instead of spotted. White river crayfish can also be found in streams and ditches with a stronger flow than what is preferred by the red swamp crayfish. -

2007 Book Predationinorganis
Ashraf M.T. Elewa Predation in Organisms A Distinct Phenomenon Ashraf M.T. Elewa Editor Predation in Organisms A Distinct Phenomenon with 48 Figures and 9 Tables Professor Ashraf M.T. Elewa Minia University Faculty of Science Department of Geology 61519 Minia Egypt Email: [email protected] Library of Congress Control Number: 2006932817 ISBN-10 3-540-46044-6 Springer Berlin Heidelberg New York ISBN-13 978-3-540-46044-2 Springer Berlin Heidelberg New York This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable to prosecution under the German Copyright Law. Springer is a part of Springer Science+Business Media springer.com © Springer-Verlag Berlin Heidelberg 2007 The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: E. Kirchner, Heidelberg Production: Almas Schimmel Typesetting: camera-ready by the editor Printed on acid-free paper 30/3141/as 5 4 3 2 1 0 Dedication This book is dedicated to my advisor: Prof. -

White River Crayfish (Procambarus Acutus) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service White River Crayfish (Procambarus acutus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, July 2015 Photo: © Keith A. Crandall. Licensed under CC BY-NC-SA 3.0. Available: http://creativecommons.org/licenses/by-nc-sa/3.0/. 1 Native Range, and Status in the United States Native Range From Benson (2015): “Southern Atlantic coast drainage from Georgia to Maine and from the Florida panhandle to Mexico; central Mississippi Valley to the upper Great Lakes drainages.” Status in the United States From Crandall (2010): “This species is known from the coastal plain and piedmont from Maine to Georgia, and from the Florida panhandle to Texas, and Minnesota to Ohio (Ghedotti 1998, Loughman 2007). Furthermore, this species has been introduced to many areas of the US, including California, Maine and Kentucky (Loughman 2007).” “California - Introduced, Connecticut - Introduced, … Maine - Introduced, Maryland - Introduced, … Rhode Island - Introduced” Means of Introductions in the United States From Benson (2015): “Probable bait bucket or aquaculture introductions.” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2015): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Protostomia Superphylum Ecdysozoa Phylum Arthropoda Subphylum Crustacea Class Malacostraca Subclass Eumalacostraca Superorder Eucarida Order Decapoda Suborder Pleocyemata Infraorder Astacidea Superfamily Astacoidea Family Cambaridae Subfamily Cambarinae Genus Procambarus Subgenus Procambarus (Ortmannicus) Species