Environmental Factors Potentially Affecting Eurycea Naufragia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kemp's Ridley Sea Turtle Headstart 08/1994 Program (NOAA Tech Memo NMFS-OPR-3) TED Regulations for Shrimp Trawls 57 FR 57348 12/04/1992 Recovery Plan - U.S
Fishing Permits Habitat Conservation Grants Fisheries Environmental Analyses Endangered Spec Search Go fish! nmlkji This si nmlkj All of NMFS Home Threatened and Endangered Species Divisions/Branches Lists What We Do Fishery Bulletins The following list of species under NMFS Fishery Quotas jurisdiction, listed as threatened or endangered, Fishery Regulations for each state and territory. Click on the state, News/Media territory or areas below to view a list of Species: National Employee 1. Southeast Region (North Carolina to Texas and Locator the Caribbean) FOIA Information 2. South Atlantic (North Carolina to Key West Public Records Florida) Request 3. Gulf of Mexico 4. Alabama 5. Florida - Atlantic Coast 6. Florida - Gulf Coast 7. Georgia 8. Louisiana 9. Mississippi 10. North Carolina 11. Puerto Rico 12. South Carolina 13. Texas 14. U.S. Virgin Islands Home · Privacy Policy · Disclaimer · About Us · Information Quality · Contact Us · Last Updated: February 2, 2010 NOAA Fisheries Office of Protected Resources OPR Home | About OPR | Species | Permits | Laws & Policies | Programs | Education | Publications Loggerhead Turtle (Caretta caretta) Species Marine Mammals Status | Taxonomy | Species Description | Habitat | Distribution | Cetaceans Population Trends | Threats | Conservation Efforts | Regulatory Overview | Pinnipeds Key Documents | More Info Marine Turtles Marine & Anadromous Fish Status Marine Invertebrates & ESA Threatened - throughout its range Plants Species of Concern Taxonomy Threatened & Endangered Kingdom: Animalia Species Phylum: Chordata Critical Habitat Maps Class: Reptilia Order: Testudines Loggerhead turtle hatchling (Caretta caretta) Family: Cheloniidae Contact OPR Photo: Mary Wozny, Broward Glossary Genus: Caretta County Florida Sea Turtle OPR Site Map Species: caretta Conservation Program Species Description Did You Know? Loggerheads were named for their relatively large heads, which support powerful jaws and enable them to feed on Search OPR hard-shelled prey, such as whelks and conch. -
![Docket No. FWS–R2–ES–2020–0048; FF09E21000 FXES11110900000 201]](https://docslib.b-cdn.net/cover/0797/docket-no-fws-r2-es-2020-0048-ff09e21000-fxes11110900000-201-150797.webp)
Docket No. FWS–R2–ES–2020–0048; FF09E21000 FXES11110900000 201]
This document is scheduled to be published in the Federal Register on 09/15/2020 and available online at federalregister.gov/d/2020-17921, and on govinfo.gov Billing Code 4333–15 DEPARTMENT OF THE INTERIOR Fish and Wildlife Service 50 CFR Part 17 [Docket No. FWS–R2–ES–2020–0048; FF09E21000 FXES11110900000 201] RIN 1018–BE78 Endangered and Threatened Wildlife and Plants; Designation of Critical Habitat for the Georgetown and Salado Salamanders AGENCY: Fish and Wildlife Service, Interior. ACTION: Proposed rule; revisions and reopening of comment period. SUMMARY: We, the U.S. Fish and Wildlife Service (Service), are revising our proposed designation of critical habitat for the Georgetown salamander (Eurycea naufragia) and Salado salamander (Eurycea chisholmensis) in Bell and Williamson Counties, Texas. Based on published genetic analyses, we are revising the distribution of the Georgetown and Salado salamanders and are adjusting previously proposed critical habitat units accordingly. We also propose changes to our description of the physical or biological features essential to the conservation of the species. We propose a total of approximately 1,519 acres (ac) (622 hectares (ha)) of critical habitat for the species in Bell and Williamson Counties, Texas. The total amount of critical habitat we are proposing for both salamanders has increased by approximately 116 ac (47 ha). The reasons for this increase are the addition of a new occupied site for the Salado salamander and refined mapping of previously proposed critical habitat units based on more precise spring locations. We also announce the availability of a draft economic analysis (DEA) of the revised proposed designation of critical habitat for the Georgetown and Salado salamanders. -

Caudata: Hynobiidae): Heterochronies and Reductions
65 (1): 117 – 130 © Senckenberg Gesellschaft für Naturforschung, 2015. 4.5.2015 Development of the bony skeleton in the Taiwan salamander, Hynobius formosanus Maki, 1922 (Caudata: Hynobiidae): Heterochronies and reductions Anna B. Vassilieva 1 *, June-Shiang Lai 2, Shang-Fang Yang 2, Yu-Hao Chang 1 & Nikolay A. Poyarkov, Jr. 1 1 Department of Vertebrate Zoology, Biological Faculty, Lomonosov Moscow State University, Leninskiye Gory, GSP-1, Moscow 119991, Russia — 2 Department of Life Science, National Taiwan Normal University, 88, Sec. 4 Tingchou Rd., Taipei 11677, Taiwan, R.O.C. — *Cor- responding author; vassil.anna(at)gmail.com Accepted 19.ii.2015. Published online at www.senckenberg.de / vertebrate-zoology on 4.v.2015. Abstract The development of the bony skeleton in a partially embryonized lotic-breeding salamander Hynobius formosanus is studied using the ontogenetic series from late embryos to postmetamorphic juveniles and adult specimen. Early stages of skull development in this spe- cies are compared with the early cranial ontogeny in two non-embryonized lentic-breeding species H. lichenatus and H. nigrescens. The obtained results show that skeletal development distinguishes H. formosanus from other hynobiids by a set of important features: 1) the reduction of provisory ossifications (complete absence of palatine and reduced state of coronoid), 2) alteration of a typical sequence of ossification appearance, namely, the delayed formation of vomer and coronoid, and 3) the absence of a separate ossification center of a lacrimal and formation of a single prefrontolacrimal. These unique osteological characters in H. formosanus are admittedly connected with specific traits of its life history, including partial embryonization, endogenous feeding until the end of metamorphosis and relatively short larval period. -

A Checklist and Annotated Bibliography of the Subterranean Aquatic Fauna of Texas
A CHECKLIST AND ANNOTATED BIBLIOGRAPHY OF THE SUBTERRANEAN AQUATIC FAUNA OF TEXAS JAMES R. REDDELL and ROBERT W. MITCHELL Texas Technological College WATER RESOURCES \ CENTER Lubbock, Texas WRC 69-6 INTERNATIONAL CENTER for ARID and August 1969 SEMI-ARID LAND STUDIES A CHECKLIST AND ANNOTATED BIBLIOGRAPHY OF THE SUBTERRANEAN AQUATIC FAUNA OF TEXAS James R. Reddell and Robert W. Mitchell Department of Biology Texas Tech University Lubbock, Texas INTRODUCTION In view of the ever-increasing interest in all studies relating to the water resources of Texas, we have found it timely to prepare this guide to the fauna and biological literature of our subterranean waters. The value of such a guide has already been demonstrated by Clark (1966) in his "Publications, Personnel, and Government Organizations Related to the Limnology, Aquatic Biology and Ichthyology of the Inland Waters of Texas". This publication dea ls primarily with inland surface waters, however, barely touching upon the now rather extensive literature which has accumulated on the biology of our subterranean waters. To state a n obvious fact, it is imperative that our underground waters receive the attention due them. They are one of our most important resources. Those subterranean waters for which biological data exi st are very un equally distributed in the state. The best known are those which are acces sible to collection and study via the entrances of caves. Even in cavernous regions there exist inaccessible deep aquifers which have yielded little in formation as yet. Biological data from the underground waters of non-cave rn ous areas are virtually non-existant. -

Salado Residents Want Better Science on Salamander
Shopping Map of Salado on pages 4B-5B Salado illageillage oiceoice VOL. XXXV, NUMBER 9V TVHUrsDAY, JUNE 21, 2012 254/947-5321 FAX 254/947-9479 V V WWW.SALADOVILLAGEVOICE.COM 50¢ Residents want better science on salamander BY TIM FLEISCHER of the Salado Creek water- line the approach to the EDITOR-IN-CHIEF shed and the water quality $60,000 year-long study and water quantity of the and to introduce the prin- As traffic on Highway habitat of the Salado sala- cipal scientists who will be 45 in Round Rock roared mander. gathering data in the com- overhead, Congressman Neither of these efforts ing months. John Carter announced are likely to impact the Meanwhile, the U.S. at a June 18 press confer- pending decision by the Fish and Wildlife Service ence his plans to attach an U.S. Fish and Wildlife Ser- (FWS) is slated to an- amendment to the Interior vice to add the Salado sala- nounce whether the four and Environment Appro- mander as an Endangered species of salamanders in priations Bill that would species. Bell, Williamson and Tra- strip any funding “to ad- While Rep. Carter said vis counties will be listed vance or pursue adding any he will propose the House as endangered. The dead- of the population of blind bill amendment within a line for FWS to propose salamanders of Salado, week, the Appropriations those species for listing is Jollyville Plateau, George- bill has many hurdles to Sept. 30. The schedule for town or Austin, Texas, to pass. First, it has to get out listing the salamander spe- the list of endangered spe- of Appropriations com- cies is part of a settlement cies or as a threatened spe- mittee (with the amend- between USFWS and the cies under the Endangered ment in place) and to the Center for Biological Di- Species Act of 1973.” House floor. -

View of the Abundance of Predators
********************************************************************* ********************************************************************* Document-ID: 2194356 Patron: Note: NOTICE: ********************************************************************* ********************************************************************* Pages: 10 Printed: 01-18-12 11:05:38 Sender: Ariel / Windows Journal Title: Texas Journal of Science 1/18/2012 7:45 AM (Please update within 24 hours) Volume: 27 Issue: 1 Call#: 01 .T4 MonthNear: 1976 Pages: 179-195 Location: evans Article Author: Tupa and Davis Not Wanted Date: 07/14/2012 Article Title: Population Dynamics of the San Marcos, Texas salamander Eurycea nana Status: T AES San Antonio Phone: 830-214-5878 Note: E-mail: [email protected] Name: Bandel, Micaela T AES San Antonio ,.. N z Address: 1- 2632 Broadway, Suite 301 South San Antonio, TX 78215 "Ccu ::::i ..J 178 THE TEXAS JOURNAL OF SC lENC£ appears to be less tolerant of arid conditions than B spec· . lOSUS and th spnng and summer of 1971 may have prevented this species f ' e dry ing in that year. rom reproduc- I wish to thank Dr. E. William Behrens for supplying the c1· data and the Port Aransas Police for their patience with my imatologica} pOPULATION DYNAMICS OF THE SAN MARCOS ditions. nocturnal expe. SALAMANDER, EURYCEA NANA BISHOP LITERATURE CITED by DIANNA DOWDEN TUPA 1 and WILLIAM K . DAVIS Blair, W. F., 1953 - Growth, dispersal, and age of sexual maturity of theM . Department of Biology, Southwest Texas State Untversity, San (Bufo val/iceps Weigmann). Copeia 1953: 20 . exican toad 8 212 Marcos 78666 Bragg, A. N. and C. C. Smith, 1943 - Observations on the ecology d 0; ~.nura. IV The ecological distribution of toads in Oklahoma~c;/~.;~~:s~1- ABSTRACT 0 Results of a study of the population ecology and demography of a paedogenetic spe Conant, R., 1958 - A Field Guide to the Reptiles and Amphibians H h . -

San Marcos Recovery Plan
~""'.- San Marcos Recovery Plan U. • Fish & Wildlife Service Albu,querque, New Mexico .-1-934--- The San Marcos Recovery Plan FOR San Hareqs River, Endangered and Threatened Species San ,Marcos Gambusia (Gambusia georgei) Hubbs and Peden ~ountainDarter (Etheostoma fonticola) (Jordan and Gilbert) San Marcos Salamander (Eurycea nana) Bish~p Te1,Ca s Wildr ice" (Z.tzaniatexana}Hitchcock PREPARED BY THE SAN MARIDS REIDVERY TEAM Dr. Robert J. Edwards, Leader, Pan American University, Edinburg, Texas 78539 Mr. Harold E. Beaty, 3414 Forest Trail, Temple, Texas 76502 Dr. Glenn Longley, Southwest Texas State University, San Marcos, Texas 78666 Mr. David H. Riskind, Texas Parks and Wildlife Department, Austin, Texas 78744 Dr. Dianna D.Tupa, center for Research and Water Resources, Unlvers1tyof Texas Balcones Research' Center, Austin, Texas 78758 Dr. Bobby G.Whites ide, Southwes t Texas State University, San Marcos, Texas 78666 OONSULTANTS Mr. Harry Bishop, USFWS, Fish Cult. & Develop. Res. Center, San Marcos, Texas 78666 Dr. W. H. P. Emery, South~est Texas State University, San Marcos, Texas 7e666 (Member 1981-82) Mr. Russell L. Masters, Edwards Underground Water District, San AntoniO, Texas 78205 Mr. William McPherson, Soil Conservation Service, Bastrop, Texas 78602 Mr. Floyd E. Potter, Jr., Texas Parks and lHl(,ili~~ Department, Austin, Texas 78759 i;:'y' , . // i f ,It . '1 ,/.' "V- Approved: '. ~ /-, j;t Regtaff'a i or, Region 2 U.S. Fi h, .. ' 'Wildlife Service Date: '/ .f --------~~~~~--------------------- SUMMARY 1 •. GOAL: Secure the survival and eventual recovery of the San Marcos .. gambusia, f01.nltain darter, San Marcos salamander, and Texas wildrice through protection of their natural ecosystem, the . San Marcos River. -
Resource Partitioning in Two Stream Salamanders, Dicamptodon Tenebrosus and Rhyacotriton Cascadae, from the Oregon Cascade Mountains
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/269579136 Resource Partitioning in Two Stream Salamanders, Dicamptodon tenebrosus and Rhyacotriton cascadae, from the Oregon Cascade Mountains Article in American Midland Naturalist · July 2014 DOI: 10.1674/0003-0031-172.1.191 CITATIONS READS 6 97 2 authors, including: R. Bruce Bury United States Geological Survey 123 PUBLICATIONS 2,940 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Herpetological Conservation and Biology View project Tailed frog phylogenetics View project All content following this page was uploaded by R. Bruce Bury on 09 February 2015. The user has requested enhancement of the downloaded file. Resource Partitioning in Two Stream Salamanders, Dicamptodon tenebrosus and Rhyacotriton cascadae, from the Oregon Cascade Mountains Author(s): Wynn W. CudmoreR. Bruce Bury Source: The American Midland Naturalist, 172(1):191-199. 2014. Published By: University of Notre Dame DOI: http://dx.doi.org/10.1674/0003-0031-172.1.191 URL: http://www.bioone.org/doi/full/10.1674/0003-0031-172.1.191 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. -
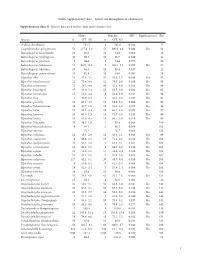
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Amphibian Assemblages in Zero-Order Basins in the Oregon Coast Range1
Color profile: Generic CMYK printer profile Composite Default screen 1452 Amphibian assemblages in zero-order basins in the Oregon Coast Range1 Chris D. Sheridan and Deanna H. Olson Abstract: Zero-order basins, extending from ridgelines to the initiation of first-order streams, were sampled in the Coast Range of Oregon to (i) characterize spatial distribution patterns of amphibian species and assemblages along lon- gitudinal and lateral gradients, and relative to three geomorphic surfaces (valleys, headmost areas, and slopes); and (ii) develop empirical species–habitat models. Unmanaged zero-order basins were hotspots for amphibian diversity, with significant differences across geomorphic gradients. Captures of riparian-associated amphibians were higher in valley areas, usually within2mofbasin center. Upland-associated amphibians were captured two times farther from basin centers than riparian-associated species, but highest densities occurred only 2–5 m from basin center. The most useful empirical models related captures of individual amphibian species to geomorphic, disturbance, moisture, and overstory variables. Ordination and indicator species analysis characterized geomorphic and other environmental gradients in am- phibian assemblages and suggested spatial compression of fluvial habitats and riparian-associated species in zero-order basins, in comparison with downstream areas. Our findings have implications for headwater areas managed to hedge risk to and uncertainty in amphibian persistence, namely in the delineation of zones with -

Phylogenetic Relationships and Systematic Revision of Central Texas Hemidactyliine Plethodontid Salamanders
HerpetologicalMonographs, 14, 2000, 1-80 ? 2000 by The Herpetologists'League, Inc. PHYLOGENETIC RELATIONSHIPS AND SYSTEMATIC REVISION OF CENTRAL TEXAS HEMIDACTYLIINE PLETHODONTID SALAMANDERS PAUL T. CHIPPINDALE,15 ANDREW H. PRICE,2 JOHN J. WIENS,3 AND DAVID M. HILLIS4 'Dept. of Biology, The University of Texas at Arlington, Arlington, TX 76019, U.S.A. 2Texas Parks and Wildlife Dept., 4200 Smith School Road, Austin, TX 78744, U.S.A. 3Carnegie Museum of Natural History, 4400 Forbes Ave., Pittsburgh, PA 15213-4080, U.S.A. 4Section of Integrative Biology and Institute for Cellular and Molecular Biology, The University of Texas at Austin, Austin, TX 78712, U.S.A. ABSTRACT: Genetic variation and phylogenetic relationships of central Texas nontransforming spring and cave salamanders, genera Eurycea and Typhlomolge (Plethodontidae: Plethodontinae: Hemidactyliini), were examined using 25 allozyme loci and DNA sequence data for a maximum of 356 bp of the mitochondrial cytochrome b gene. Monophyly of the central Texas hemidactyliines is well supported. High levels of divergence occur among many populations and groups of populations, and there clearly are many more species in the group than previously recognized. Many have extremely restricted distributions in isolated islands of aquatic habitat. Several major monophyletic groups were identified that correspond to geographically circumscribed areas of the Edwards Plateau region. The deepest phylogenetic split in the group occurs between populations northeast versus southwest of the Colorado River. Species that have been assigned to the genus Typhlomolge are phylogenetically nested within the central Texas Eurycea; therefore, the genus Typhlomolge is placed in the synonymy of Eurycea. Continued recognition of the species E. -

Mesohabitat Associations of the Threatened San Marcos Salamander (Eurycea Nana) Across Its Geographic Range
AQUATIC CONSERVATION: MARINE AND FRESHWATER ECOSYSTEMS Aquatic Conserv: Mar. Freshw. Ecosyst. 25: 307–321 (2015) Published online 19 March 2015 in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/aqc.2559 Mesohabitat associations of the threatened San Marcos salamander (Eurycea nana) across its geographic range PETER H. DIAZa, JOE N. FRIESb, TIMOTHY H. BONNERa, MARA L. ALEXANDERb and WESTON H. NOWLINa,* aDepartment of Biology, Aquatic Station, Texas State University, San Marcos, Texas 78666, USA bSan Marcos Aquatic Resource Center, United States Fish and Wildlife Service, San Marcos Texas 78666, USA ABSTRACT 1. Habitat loss is one of the most critical factors affecting the loss of species. However, habitat conservation of many threatened species is performed with incomplete information on habitat requirements and trophic ecology, thus presenting a challenge to designing and implementing recovery plans. 2. The San Marcos salamander (Eurycea nana) is a federally threatened spring-associated organism whose geographic distribution is limited to the headwaters of the San Marcos River in Texas, USA. Although its designated critical habitat includes the headwaters and the first 50 m of the river, little is known of its habitat requirements or co-occurrence with benthic macroinvertebrates and macrophytes. 3. This study examined mesohabitat associations of the salamander and patterns of co-occurrence with macrophytes and benthic invertebrates within its critical habitat. Surveys of mesohabitat characteristics were conducted during a one-year period and data were analysed to assess mesohabitat associations of the San Marcos salamander and patterns of co-occurrence with invertebrates and macrophytes. 4. Salamanders were distributed throughout the critical habitat, but were almost exclusively found in mesohabitats containing cobble and gravel with coverage of Amblystegium and filamentous algae.