Overexpression of Kru¨Ppel-Like Factor 4 in the Human Colon Cancer Cell Line RKO Leads to Reduced Tumorigenecity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Keratin 19 Regulates Cell Shape and Cell-Cell Adhesion of MCF7 Cells While Maintaining E-Cadherin Localization at the Cell Surface
Keratin 19 regulates cell shape and cell-cell adhesion of MCF7 cells while maintaining E-cadherin localization at the cell surface Welcome to my poster. This is Sarah Alsharif, a PhD student from the biology department. I am glad to present the work our lab has been doing in the breast cancer field. In fact, after lung cancer, breast cancer is the second cause of death in women worldwide (1). It is estimated that every 18 seconds, approximately one new case of breast cancer is documented (2). No one dies due to cancer itself. The death is because of metastasis which takes place when cancer cells leave a breast in which they are formed and reach other sites such as the brain or lung. Our lab is interested in investigating the mechanism behind metastasis of breast cancer. Metastasis is associated with what is called epithelial to mesenchymal transition (EMT), the process characterized by loss of cell to cell adhesion and expression of epithelial markers such as keratin intermediate filament proteins, as you can see in the first three images of cells. Those filaments are keratins and they are critical for the shape and for maintaining mechanical integrity of epithelial cells via cell to cell complexes called desmosomes. Among different keratins, keratin 19 (K19) is highly expressed in many types of cancer including breast cancer, and is correlated with a worse prognosis (3). Consistently, K19 expression has been reported to be significantly higher in metastatic breast cancer tumor cells compared to primary tumors (4). The role of K19 on mechanical properties of cancer cells for cell migration and possible impact on metastasis in breast cancer patients is still unknown. -

Quantigene Flowrna Probe Sets Currently Available
QuantiGene FlowRNA Probe Sets Currently Available Accession No. Species Symbol Gene Name Catalog No. NM_003452 Human ZNF189 zinc finger protein 189 VA1-10009 NM_000057 Human BLM Bloom syndrome VA1-10010 NM_005269 Human GLI glioma-associated oncogene homolog (zinc finger protein) VA1-10011 NM_002614 Human PDZK1 PDZ domain containing 1 VA1-10015 NM_003225 Human TFF1 Trefoil factor 1 (breast cancer, estrogen-inducible sequence expressed in) VA1-10016 NM_002276 Human KRT19 keratin 19 VA1-10022 NM_002659 Human PLAUR plasminogen activator, urokinase receptor VA1-10025 NM_017669 Human ERCC6L excision repair cross-complementing rodent repair deficiency, complementation group 6-like VA1-10029 NM_017699 Human SIDT1 SID1 transmembrane family, member 1 VA1-10032 NM_000077 Human CDKN2A cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) VA1-10040 NM_003150 Human STAT3 signal transducer and activator of transcripton 3 (acute-phase response factor) VA1-10046 NM_004707 Human ATG12 ATG12 autophagy related 12 homolog (S. cerevisiae) VA1-10047 NM_000737 Human CGB chorionic gonadotropin, beta polypeptide VA1-10048 NM_001017420 Human ESCO2 establishment of cohesion 1 homolog 2 (S. cerevisiae) VA1-10050 NM_197978 Human HEMGN hemogen VA1-10051 NM_001738 Human CA1 Carbonic anhydrase I VA1-10052 NM_000184 Human HBG2 Hemoglobin, gamma G VA1-10053 NM_005330 Human HBE1 Hemoglobin, epsilon 1 VA1-10054 NR_003367 Human PVT1 Pvt1 oncogene homolog (mouse) VA1-10061 NM_000454 Human SOD1 Superoxide dismutase 1, soluble (amyotrophic lateral sclerosis 1 (adult)) -
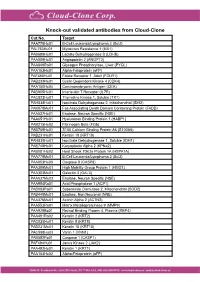
Knock-Out Validated Antibodies from Cloud-Clone Cat.No
Knock-out validated antibodies from Cloud-Clone Cat.No. Target PAA778Hu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAL763Hu01 Myxovirus Resistance 1 (MX1) PAB698Hu01 Lactate Dehydrogenase B (LDHB) PAA009Hu01 Angiopoietin 2 (ANGPT2) PAA849Ra01 Glycogen Phosphorylase, Liver (PYGL) PAA153Hu01 Alpha-Fetoprotein (aFP) PAF460Hu01 Folate Receptor 1, Adult (FOLR1) PAB233Hu01 Cyclin Dependent Kinase 4 (CDK4) PAA150Hu04 Carcinoembryonic Antigen (CEA) PAB905Hu01 Interleukin 7 Receptor (IL7R) PAC823Hu01 Thymidine Kinase 1, Soluble (TK1) PAH838Hu01 Isocitrate Dehydrogenase 2, mitochondrial (IDH2) PAK078Mu01 Fas Associating Death Domain Containing Protein (FADD) PAA537Hu01 Enolase, Neuron Specific (NSE) PAA651Hu01 Hyaluronan Binding Protein 1 (HABP1) PAB215Hu02 Fibrinogen Beta (FGb) PAB769Hu01 S100 Calcium Binding Protein A6 (S100A6) PAB231Hu01 Keratin 18 (KRT18) PAH839Hu01 Isocitrate Dehydrogenase 1, Soluble (IDH1) PAE748Hu01 Karyopherin Alpha 2 (KPNa2) PAB081Hu02 Heat Shock 70kDa Protein 1A (HSPA1A) PAA778Mu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAA853Hu03 Caspase 8 (CASP8) PAA399Mu01 High Mobility Group Protein 1 (HMG1) PAA303Mu01 Galectin 3 (GAL3) PAA537Mu02 Enolase, Neuron Specific (NSE) PAA994Ra01 Acid Phosphatase 1 (ACP1) PAB083Ra01 Superoxide Dismutase 2, Mitochondrial (SOD2) PAB449Mu01 Enolase, Non Neuronal (NNE) PAA376Mu01 Actinin Alpha 2 (ACTN2) PAA553Ra01 Matrix Metalloproteinase 9 (MMP9) PAA929Bo01 Retinol Binding Protein 4, Plasma (RBP4) PAA491Ra02 Keratin 2 (KRT2) PAC025Hu01 Keratin 8 (KRT8) PAB231Mu01 Keratin 18 (KRT18) PAC598Hu03 Vanin 1 (VNN1) -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7 -

Toward Unraveling the Complexity of Simple Epithelial Keratins in Human Disease
Toward unraveling the complexity of simple epithelial keratins in human disease M. Bishr Omary, … , Pavel Strnad, Shinichiro Hanada J Clin Invest. 2009;119(7):1794-1805. https://doi.org/10.1172/JCI37762. Review Series Simple epithelial keratins (SEKs) are found primarily in single-layered simple epithelia and include keratin 7 (K7), K8, K18–K20, and K23. Genetically engineered mice that lack SEKs or overexpress mutant SEKs have helped illuminate several keratin functions and served as important disease models. Insight into the contribution of SEKs to human disease has indicated that K8 and K18 are the major constituents of Mallory-Denk bodies, hepatic inclusions associated with several liver diseases, and are essential for inclusion formation. Furthermore, mutations in the genes encoding K8, K18, and K19 predispose individuals to a variety of liver diseases. Hence, as we discuss here, the SEK cytoskeleton is involved in the orchestration of several important cellular functions and contributes to the pathogenesis of human liver disease. Find the latest version: https://jci.me/37762/pdf Review series Toward unraveling the complexity of simple epithelial keratins in human disease M. Bishr Omary,1 Nam-On Ku,1,2 Pavel Strnad,3 and Shinichiro Hanada1,4 1Department of Molecular & Integrative Physiology, University of Michigan Medical School, Ann Arbor, Michigan, USA. 2Department of Biomedical Sciences, Graduate School, Yonsei University, Seoul, Republic of Korea. 3Department of Internal Medicine I, University Medical Center Ulm, Ulm, Germany. 4Division of Gastroenterology, Department of Medicine, Kurume University School of Medicine, Kurume, Japan. Simple epithelial keratins (SEKs) are found primarily in single-layered simple epithelia and include keratin 7 (K7), K8, K18–K20, and K23. -

Effector Gene Expression Potential to Th17 Cells by Promoting Microrna
Downloaded from http://www.jimmunol.org/ by guest on September 26, 2021 is online at: average * The Journal of Immunology published online 17 May 2013 from submission to initial decision 4 weeks from acceptance to publication http://www.jimmunol.org/content/early/2013/05/17/jimmun ol.1300351 MicroRNA-155 Confers Encephalogenic Potential to Th17 Cells by Promoting Effector Gene Expression Ruozhen Hu, Thomas B. Huffaker, Dominique A. Kagele, Marah C. Runtsch, Erin Bake, Aadel A. Chaudhuri, June L. Round and Ryan M. O'Connell J Immunol Submit online. Every submission reviewed by practicing scientists ? is published twice each month by http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://www.jimmunol.org/content/suppl/2013/05/17/jimmunol.130035 1.DC1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 26, 2021. Published May 17, 2013, doi:10.4049/jimmunol.1300351 The Journal of Immunology MicroRNA-155 Confers Encephalogenic Potential to Th17 Cells by Promoting Effector Gene Expression Ruozhen Hu,* Thomas B. Huffaker,* Dominique A. Kagele,* Marah C. Runtsch,* Erin Bake,* Aadel A. Chaudhuri,† June L. -

Alterations in Keratin Levels and Modifications in Colorectal Adenomagenesis Ria Rosser
Alterations in Keratin Levels and Modifications in Colorectal Adenomagenesis Ria Rosser January 2016 Supervisors: BM Corfe and KS Chapple Department of Oncology Thesis submitted to the University of Sheffield for the degree of Doctor of Medicine 2 Alterations in Keratin Levels and Modifications in Colorectal Adenomagenesis Table of Contents Abstract ................................................................................................................................. 7 Acknowledgments ............................................................................................................. 9 List of Figures ................................................................................................................... 11 List of Tables .................................................................................................................... 13 Abbreviations .................................................................................................................. 15 Chapter 1 Literature review ....................................................................................... 19 1.1 The History of Adenomatous polyps ................................................................. 19 1.1.1 Origins of the Adenoma ............................................................................................... 20 1.1.2 Adenoma-Carcinogenesis model ......................................................................................... 24 1.2 Field effects – Theory and Definitions ............................................................. -

View Board (IRB) at Tuskegee University
Myers et al. BMC Cancer (2017) 17:480 DOI 10.1186/s12885-017-3462-7 RESEARCH ARTICLE Open Access Proteomic characterization of paired non- malignant and malignant African-American prostate epithelial cell lines distinguishes them by structural proteins Jennifer S. Myers1, Karin A. Vallega1, Jason White2, Kaixian Yu3, Clayton C. Yates2 and Qing-Xiang Amy Sang1* Abstract Background: While many factors may contribute to the higher prostate cancer incidence and mortality experienced by African-American men compared to their counterparts, the contribution of tumor biology is underexplored due to inadequate availability of African-American patient-derived cell lines and specimens. Here, we characterize the proteomes of non-malignant RC-77 N/E and malignant RC-77 T/E prostate epithelial cell lines previously established from prostate specimens from the same African-American patient with early stage primary prostate cancer. Methods: In this comparative proteomic analysis of RC-77 N/E and RC-77 T/E cells, differentially expressed proteins were identified and analyzed for overrepresentation of PANTHER protein classes, Gene Ontology annotations, and pathways. The enrichment of gene sets and pathway significance were assessed using Gene Set Enrichment Analysis and Signaling Pathway Impact Analysis, respectively. The gene and protein expression data of age- and stage-matched prostate cancer specimens from The Cancer Genome Atlas were analyzed. Results: Structural and cytoskeletal proteins were differentially expressed and statistically overrepresented between RC-77 N/E and RC-77 T/E cells. Beta-catenin, alpha-actinin-1, and filamin-A were upregulated in the tumorigenic RC-77 T/E cells, while integrin beta-1, integrin alpha-6, caveolin-1, laminin subunit gamma-2, and CD44 antigen were downregulated. -

Terrestrial Vertebrates Have Two Keratin Gene Clusters; Striking Differences in Teleost fish Alexander Zimek, Klaus Weberã
ARTICLE IN PRESS European Journal of Cell Biology 84 (2005) 623–635 www.elsevier.de/ejcb Terrestrial vertebrates have two keratin gene clusters; striking differences in teleost fish Alexander Zimek, Klaus Weberà Department of Biochemistry and Cell Biology, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, D-37077 Go¨ttingen, Germany Received 16 December 2004; received in revised form 25 January 2005; accepted 25 January 2005 Abstract Keratins I and II form the largest subgroups of mammalian intermediate filament (IF) proteins and account as obligatory heteropolymers for the keratin filaments of epithelia. All human type I genes except for the K18 gene are clustered on chromosome 17q21, while all type II genes form a cluster on chromosome 12q13, that ends with the type I gene K18. Highly related keratin gene clusters are found in rat and mouse. Since fish seem to lack a keratin II cluster we screened the recently established draft genomes of a bird (chicken) and an amphibian (Xenopus). The results show that keratin I and II gene clusters are a feature of all terrestrial vertebrates. Because hair with its multiple hair keratins and inner root sheath keratins is a mammalian acquisition, the keratin gene clusters of chicken and Xenopus tropicalis have only about half the number of genes found in mammals. Within the type I clusters all genes have the same orientation. In type II clusters there is a rare gene of opposite orientation. Finally we show that the genes for keratins 8 and 18, which are the first expression pair in embryology, are not only adjacent in mammals, but also in Xenopus and three different fish. -

Rho Gtpase Signalling Pathways in the Morphological Changes Associated with Apoptosis
Cell Death and Differentiation (2002) 9, 493 ± 504 ã 2002 Nature Publishing Group All rights reserved 1350-9047/02 $25.00 www.nature.com/cdd Review Rho GTPase signalling pathways in the morphological changes associated with apoptosis ML Coleman1 and MF Olson*,1 membrane blebs and apoptotic bodies (reviewed in refer- ence3). Ultimately, the dead cell is packaged into membrane- 1 Cancer Research Campaign Centre for Cell and Molecular Biology, Institute of clad apoptotic bodies that facilitate uptake by neighbouring Cancer Research, 237 Fulham Road, London SW3 6JB, UK cells or by specialised phagocytic cells. Dynamic re- * Corresponding author: MF Olson, Abramson Family Cancer Research arrangements of the actin cytoskeleton in the phagocyte are Institute, Room 411, BRB II/III, 421 Curie Boulevard, University of required for the internalisation of apoptotic cell fragments. Pennsylvania, Philadelphia, PA 19104-6160, USA. Tel: +1 215 746 6798; Fax: +1 215 746 5525; E-mail: [email protected] Recent research has revealed the importance of signal transduction pathways controlled by Rho family GTPases in Received 23.8.01; revised 26.10.01; accepted 5.11.01 regulating the marked changes in cell morphology observed Edited by M Piacentini in the processes of apoptosis and phagocytosis. The Rho GTPases are a family of proteins (RhoA, RhoB, RhoC, RhoD, RhoE/Rnd3, RhoG, RhoH/TTF, Rnd1, Rnd2, Abstract Rac1, Rac2, Rac3, Cdc42/G25K, Wrch-1, TC10, TCL, Chp, Rif) that act as molecular switches in intracellular signal The killing and removal of superfluous cells is an important 4 step during embryonic development, tissue homeostasis, transduction pathways (reviewed in reference ). -

Discerning the Role of Krüppel-Like Factor 4 in Breast Cancer
DISCERNING THE ROLE OF KRÜPPEL-LIKE FACTOR 4 IN BREAST CANCER by JENNIFER L. YORI Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Dr. Ruth A. Keri Department of Pharmacology CASE WESTERN RESERVE UNIVERSITY May, 2011 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of ______________________________________________________ candidate for the ________________________________degree *. (signed)_______________________________________________ (chair of the committee) ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ (date) _______________________ *We also certify that written approval has been obtained for any proprietary material contained therein. DEDICATION This work is dedicatied to Beverly Ann McMillan for all that she taught me and Fiona Campbell Yori for all that she teaches me everyday. iii TABLE OF CONTENTS List of Tables vii List of Figures viii Acknowledgements x Abbreviations xii Abstract 1 Chapter I INTRODUCTION, REVIEW OF THE LITERTURE, AND 3 STATEMENT OF PURPOSE 1.1 Molecular/Histopathological subtypes and cellular origin of 3 breast cancer 1.1.1 Luminal breast cancer 5 1.1.2 HER2/ERRB2 breast cancer 6 1.1.3 Basal-like breast cancer 8 1.1.4 Comparison of mouse models and human tumors generate a 10 new molecular subtype 1.2