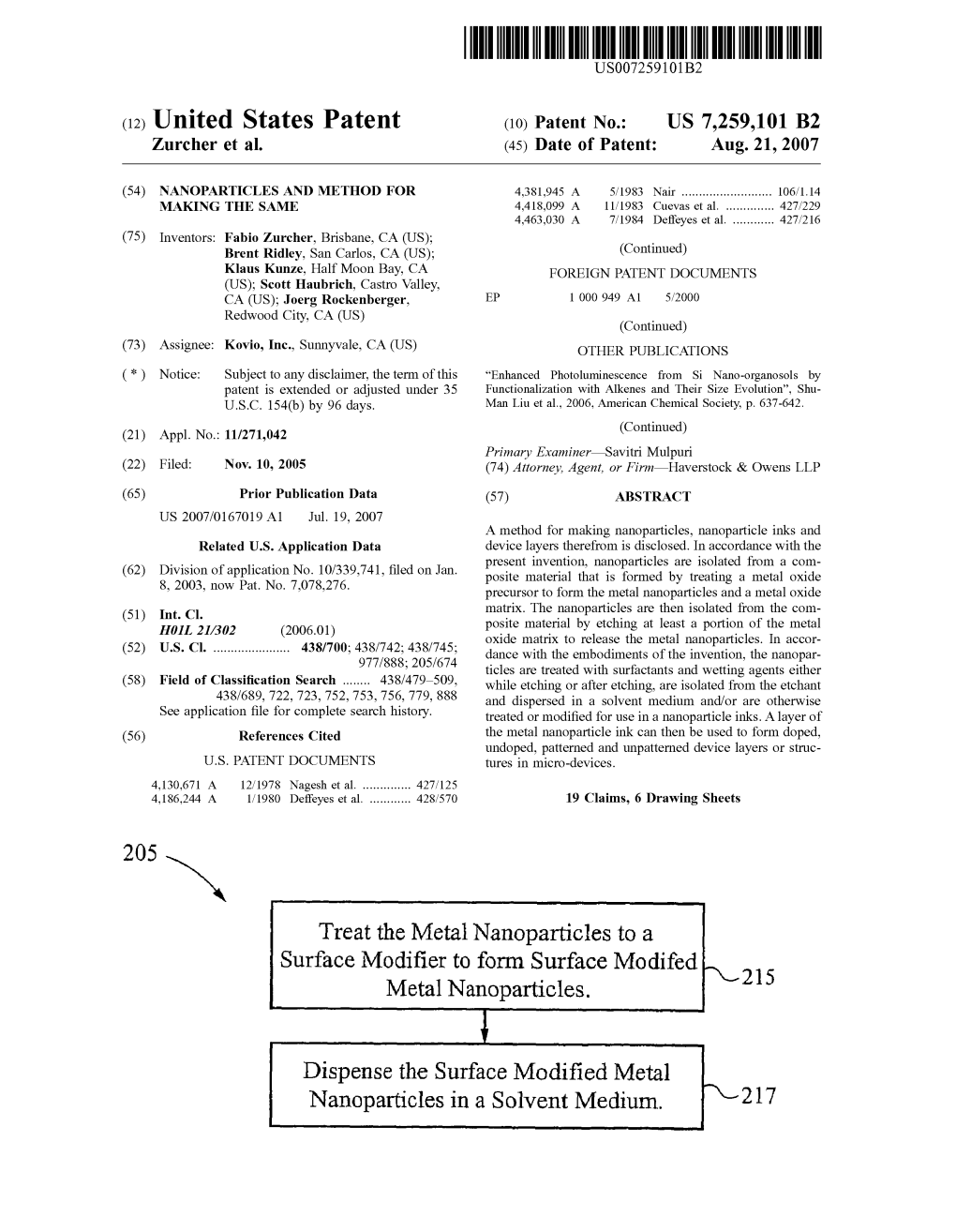
Treat the Metal Nanoparticles to a Surface Modifier to Form Surface Modified Metal Nanoparticles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

Peculiarities of the Thermo-Optic Coefficient at High Temperatures in Fibers Containing Bragg Gratings
PECULIARITIES OF THE THERMO-OPTIC COEFFICIENT AT HIGH TEMPERATURES IN FIBERS CONTAINING BRAGG GRATINGS A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Igor Fedin August, 2011 PECULIARITIES OF THE THERMO-OPTIC COEFFICIENT AT HIGH TEMPERATURES IN FIBERS CONTAINING BRAGG GRATINGS Igor Fedin Thesis Approved: Accepted: ______________________________ ______________________________ Advisor Dean of the College Dr. Sergei F. Lyuksyutov Dr. Chand K. Midha ______________________________ ______________________________ Faculty Reader Dean of the Graduate School Dr. David S. Perry Dr. George R. Newkome ______________________________ ______________________________ Department Chair Date Dr. Robert R. Mallik ii ABSTRACT The temperature dependence of thermo-optic coefficient in silica-based fibers containing fiber Bragg gratings (FBGs) includes thermal instability of chemical composition gratings, non-linear temperature dependence of FBGs written in different fibers, quadratic behavior of FBGs, and long-term stability of silica-based FBGs. Experimental measurements of the thermo-optic coefficient for the temperature interval 50 – 7800C in fused silica fiber containing FBGs were conducted while the temperature shift of the Bragg’s peak was monitored between 1300 and 1311 nm with sub-Angstrom precision. Numerical computations were focused on the FBG’s diffraction efficiency calculations accounting for the temperature drift of the gratings and found to be in excellent agreement with obtained experimental data. It has been found that the thermo- optic coefficient changes between 0.79×10-5 and 1.45×10-5 K-1 and undergoes a minimum in the vicinity of 440°C. Additional observation indicates a negative sign of the second- order thermo-optic coefficient. -

Euroresearch & Consulting
Euroresearch & Consulting LLC Market Research: Global Market for Germanium and Germanium Products. 1 CONTENTS CHAPTER 1. GENERAL REVIEW OF THE INDUSTRY. ......................................................... 5 1.1. HISTORIC, PRESENT AND ESTIMATED CORRELATION OF GLOBAL GERMANIUM DEMAND AND SUPPLY. ................................................................................................................... 5 1.2. ANALYSIS OF PRICES ON GERMANIUM............................................................................ 13 1.3. GENERAL SIZE OF THE MARKET AND PRINCIPAL SEGMENTS OF GERMANIUM CONSUMPTION AND GERMANIUM CONTAINING PRODUCTS. ........................................... 31 1.4. THE MAIN TRENDS OF GERMANIUM PRODUCTION AND CONSUMPTION. ............. 38 1.5. THE PRINCIPAL FACTORS HAMPERING INDUSTRY DEVELOPMENT AND MARKET GROWTH. .......................................................................................................................................... 47 1.6. THE ANALYSIS OF THE IMPACT OF ACTUAL AND POTENTIAL ECONOMIC SANCTIONS ON THE INDUSTRY. ................................................................................................. 48 CHAPTER 2. THE PRINCIPAL FIELDS OF GERMANIUM APPLICATION: ANALYSIS OF MAJOR TRENDS AND AVENUES. ........................................................................................... 50 CHAPTER 3. THE ANALYSIS OF THE INDUSTRY OF PRODUCTION OF GERMANIUM AND ITS DERIVATIVES ACCORDING TO PORTER 5 COMPETITIVE FORCES MODEL. .............................................................................................................................. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Bibliography on the High Temperature Chemistry and Physics of Materials
' National Bureau of Stsndards A- -' >'•' A UNITED STATES DEPARTMENT OF Library, E-01 Admin. BIdg. COMMERCE PUBLICATION :DT 1 1969 NATIONAL BUREAU OF STANDARDS SPECIAL PUBLICATION 315-2 BIBLIOGRAPHY ON THE HIGH TEMPERATURE CHEMISTRY AND PHYSICS OF MATERIALS APRIL, MAY, JUNE 1969 NATIONAL BUREAU OF STANDARDS ' The National Bureau of Standards was established by an act of Congress March 3, 1901 . Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, -

S.6 Chemistry of Aluminium, Group IV Group VII.Pdf
ALUMINIUM Atomic number 13 Electronic configuration: 1s22s22p63s23p1 Occurrence It is found in ores such as bauxite (impure hydrated aluminium oxide), Al2O3.2H2O and cryolite (impure sodium hexafluoroaluminate), Na3AlF6.It is found widely in the form of hydrated aluminium silicates in the rocks such as clays, micas and fieldspars. An ore is a naturally occurring substance from which an element is extracted. EXTRACTION OF ALUMINIUM Bauxiteis the only ore from which it is profitable to extract aluminium. The extraction of aluminium from bauxite involves two stages namely (i) Concentration (purification /dressing) of the ore. This involves removal of impurities from the ore. The main impurities in bauxite are iron(III) oxide, Fe2O3 and silicon(IV) oxide (silicon dioxide or silica), SiO2. (ii) Reduction of purified ore to the metal by electrolysis Purification (concentration) of the bauxite Bauxite is first roasted in air to convert any iron(II) oxide to iron(III) oxide and to remove water from it. The roasted ore is then pulverized (ground or crushed into a fine powder) and heated with excess concentrated sodium hydroxide solution under pressure. Aluminium oxide and silicon dioxide impurity dissolve in the alkali to form sodium aluminate and sodium silicate in the solution. The iron (III) oxide impurity does not dissolve and so is filtered off. - Al O2 (s) +2푂̅H(aq) AlO2 (aq)+ H2O (l) - orAl2O3 (s) + 2푂̅H (aq) + 3H2O(l) 2Al(OH)4 (aq) 3- orAl2O3 (s) + 6 푂̅H(aq) + 3H2O(l) 2Al(OH)6 (aq) 2- and SiO2 (s) + 2푂̅H (aq) SiO3 (aq) + H2O (l) The filtrate which contain sodium aluminate and sodium silicate is then seeded with a little solid pure aluminium hydroxide (ie a little aluminium hydroxide crystals are added to the filtrate) to precipitate out aluminium hydroxide leaving sodium silicate in solution. -

United States Patent (19) 11 3,970,745 Takeuchi (45) July 20, 1976
United States Patent (19) 11 3,970,745 Takeuchi (45) July 20, 1976 (54) METHOD FOR PRODUCING HYDROGEN son, vol. 3, 1949 Ed., p. 535, Reinhold Pub. Corp., FROM WATER New York. 75) Inventor: Norio Takeuchi, Isehara, Japan P. Pascal's “Nouveau Traite' de Chimie Minerale', Tome 8, 1963, pp. 54, 55; Masson et Cie., Paris, 73 Assignee: Director-General of the Agency of France. Industrial Science and Technology, J. W. Mellor's "A Comp. Treatise on Inorg. and Theo. Tokyo, Japan Chem.", vol. 7, Longmans, Green & Co., N. Y. 22 Filed: Mar. 24, 1975 Primary Examiner-Edward Stern (21 Appl. No.: 561,161 Attorney, Agent, or Firm-William J. Daniel 30 Foreign Application Priority Data 57 ABSTRACT May 9, 1974 Japan................................ 49-51.538 A method for producing hydrogen from water in May 17, 1974 Japan................................ 49-54504 which hydrogen is produced by reducing water using metallic germanium and the by-product, germanium 52 U.S. Cl................................... 423/657; 423193; monoxide, is reduced with a reducing agent to metal 423/532; 423/539; 423/655; 4231592 lic germanium which is recycled and reused. In this 51) Int. C.’.................so e s so C01B 1/07; COB 1102; case, it is necessary to regulate the oxidation of metal CO1G 7700 lic germanium so as to stop at the germanium monox (58) Field of Search............. 423/657, 539, 532, 93, ide stage since the reduction of germanium dioxide to 423/592 metallic germanium is difficult. Therefore, water 56) References Cited vapor is advantageously reduced by using not less than 1 mole of metallic germanium to 1 mole of water OTHER PUBLICATIONS vapor or it is indirectly reduced with an intermediate "Comp. -

Polyether Complexes of Groups 13 and 14
Chemical Society Reviews Polyether Complexes of Groups 13 and 14 Journal: Chemical Society Reviews Manuscript ID CS-REV-12-2015-000934.R1 Article Type: Review Article Date Submitted by the Author: 29-Mar-2016 Complete List of Authors: Swidan, Ala'aeddeen; University of Windsor, Department of Chemistry and Biochemistry Macdonald, Charles; University of Windsor, Department of Chemistry and Biochemistry Page 1 of 93 Chemical Society Reviews Polyether Complexes of Groups 13 and 14 Ala'aeddeen Swidan and Charles L. B. Macdonald* Department of Chemistry and Biochemistry, University of Windsor, 401 Sunset Ave. Windsor, ON, N9B 3P4, Canada Phone: 1-519-253-3000 ext.3991 Email: [email protected] Abstract Notable aspects of the chemistry of complexes of polyether ligands including crown ethers, cryptands, glycols, glymes, and related polyether ligands with heavier group 13 and 14 elements are reviewed with a focus on results from 2005 to the present. The majority of reported polyether complexes contain lead(II) and thallium(I) but recent breakthroughs in regard to the preparation of low oxidation state reagents of the lighter congeners have allowed for the generation of complexes containing indium(I), gallium(I), germanium(II), and even silicon(II). The important roles of ligand size, donor types, and counter anions in regard to the chemical properties of the polyether complexes is highlighted. A particular focus on the structural aspects of the numerous coordination complexes provides a rationale for some of the spectacular contributions that such compounds have made to Modern Main Group Chemistry . Keywords Crown ethers, polyethers, cryptands, podands, group 13, group 14, triel, tetrel, low oxidation state, low valent, coordination chemistry Chemical Society Reviews Page 2 of 93 1. -

Water Soluble Nano-Scale Transient Material Germanium Oxide for Zero Toxic Waste Based Environmentally Benign Nano-Manufacturing
Water soluble nano-scale transient material germanium oxide for zero toxic waste based environmentally benign nano-manufacturing Item Type Article Authors Almuslem, A. S.; Hanna, Amir; Yapici, Tahir; Wehbe, N.; Diallo, Elhadj; Kutbee, Arwa T.; Bahabry, Rabab R.; Hussain, Muhammad Mustafa Citation Almuslem AS, Hanna AN, Yapici T, Wehbe N, Diallo EM, et al. (2017) Water soluble nano-scale transient material germanium oxide for zero toxic waste based environmentally benign nano- manufacturing. Applied Physics Letters 110: 074103. Available: http://dx.doi.org/10.1063/1.4976311. Eprint version Publisher's Version/PDF DOI 10.1063/1.4976311 Publisher AIP Publishing Journal Applied Physics Letters Rights Archived with thanks to Applied Physics Letters Download date 24/09/2021 18:56:53 Link to Item http://hdl.handle.net/10754/622963 Water soluble nano-scale transient material germanium oxide for zero toxic waste based environmentally benign nano-manufacturing A. S. Almuslem, A. N. Hanna, T. Yapici, N. Wehbe, E. M. Diallo, A. T. Kutbee, R. R. Bahabry, and M. M. Hussain Citation: Appl. Phys. Lett. 110, 074103 (2017); doi: 10.1063/1.4976311 View online: http://dx.doi.org/10.1063/1.4976311 View Table of Contents: http://aip.scitation.org/toc/apl/110/7 Published by the American Institute of Physics Articles you may be interested in Zinc tin oxide metal semiconductor field effect transistors and their improvement under negative bias (illumination) temperature stress Appl. Phys. Lett. 110, 073502073502 (2017); 10.1063/1.4976196 Room-temperature vertical-cavity surface-emitting lasers at 4 µm with GaSb-based type-II quantum wells Appl. -

Nol Steam Reforming Thin Film Model Catalysts by (HR)TEM
Materials of Chemistry and Physics 122 (2010) 263-269 Preparation and structural characterization of SnO2 and GeO2 metha- nol steam reforming thin film model catalysts by (HR)TEM H. Lorenz1, Q. Zhao1, S. Turner2, O. I. Lebedev2, G. Van Tendeloo2, B. Klötzer1, C. Rameshan1,3, S. Penner1,* [a]Laboratory of Industrial Chemistry, Ruhr University Bochum, D-44780 Bochum, Germany. [b]Institute of Geology, Mineralogy & Geophysics, Ruhr-University Bochum, D-44780 Bochum, Germany [c]Fritz-Haber-Institut der Max-Planck-Gesellschaft, Departement of Inorganic Chemistry, D-14195 Berlin, Germany * Corresponding author: e-mail [email protected], Received 21 October 2009; revised 23 December 2009; accepted 23 March 2010. Available online 17 April 2010. Abstract Structure, morphology and composition of different tin oxide and germanium oxide thin film catalysts for the methanol steam reforming (MSR) reaction have been studied by a combination of (High-resolution) Transmission Electron Microscopy, Selected Area Electron Diffraction, Dark- field imaging and Electron Energy-Loss Spectroscopy. Deposition of the thin films on NaCl(001) cleavage faces has been carried out by thermal evaporation of the respective SnO2 and GeO2 powders in varying oxygen partial pressures and at different substrate temperatures. Preparation of tin oxide films in high oxygen pressures (10-3 mbar) exclusively resulted in SnO phases, at and above 473 K substrate temperature epitaxial growth of SnO on NaCl(001) leads to well-ordered films. For lower oxygen partial pressures (10-4-10-5 mbar), mixtures of SnO and β-Sn are obtained. Well-ordered SnO2 films, as verified by electron diffraction patterns and energy loss spectra, are only obtained after post-oxidation of SnO films at temperatures T ≥ 673 K in 1 bar O2. -

Chemicals & Reagents Selection for the Battery Industry
Chemicals & Reagents Selection for the Battery Industry Anode Formulation Materials Ref. Description Pack S Supplier 10336090 Graphite, Pure, Powder 1 kg Fisher Chemical 10380394 Graphite, powder 1 kg Acros Organics 15416055 Graphite powder, natural, high purity, -200 mesh, 99.9999% (metals basis) 28 g Alfa Aesar 11311188 Graphite powder, microcrystalline, -325 mesh, 75-82% C, 18-25% Ash 1 kg Alfa Aesar 11321188 Graphite powder, microcrystalline, -325 mesh, 75-82% C, 18-25% Ash 250 g Alfa Aesar 15432647 Graphite powder, synthetic, APS 7-11 micron, 99% 1 kg Alfa Aesar 15422647 Graphite powder, synthetic, APS 7-11 micron, 99% 25 g Alfa Aesar 11347068 Graphite powder, natural, briquetting grade, -100 mesh, 99.9995% (metals basis) 454 g Alfa Aesar 11337068 Graphite powder, natural, briquetting grade, -100 mesh, 99.9995% (metals basis) 113 g Alfa Aesar 11335077 Graphite powder, natural, briquetting grade, -200 mesh, 99.9995% (metals basis) 454 g Alfa Aesar 11355077 Graphite powder, natural, briquetting grade, -200 mesh, 99.9995% (metals basis) 113 g Alfa Aesar 11387068 Graphite powder, natural, microcrystal grade, APS 2-15 micron, 99.9995% (metals basis) 454 g Alfa Aesar 11377068 Graphite powder, natural, microcrystal grade, APS 2-15 micron, 99.9995% (metals basis) 113 g Alfa Aesar 11305087 Graphite powder, synthetic, conducting grade, -200 mesh, 99.9995% (metals basis) 454 g Alfa Aesar 11375077 Graphite powder, synthetic, conducting grade, -200 mesh, 99.9995% (metals basis) 113 g Alfa Aesar 11365087 Graphite powder, natural, universal grade, -

T. Don Tilley's Publications
Titanium-Germoxy Precursor Route to Germanium-Modified Epoxidation Catalysts with 387. Enhanced Activity. P. J. Cordeiro, P. Guillo, C. S. Spanjers, J. W. Chang, M. E. Fasulo, R. M. Rioux and T. D. Tilley, ACS Catal. 2013, 3, 2269. Photocatalytic Water Oxidation by Very Small Cobalt Domains on a Silica Surface. H. S. 386. Ahn, J. Yano and T. D. Tilley, Energy Environ. Sci. 2013, 6, 3080. Platinum-Catalyzed C-C Activation Induced by C-H Activation. M. Bowring, R. G. 385. Bergman and T. D. Tilley, J. Am. Chem. Soc. 2013, 135, 13121. Structural and mechanistic investigation of a cationic hydrogen-substituted ruthenium 384. silylene catalyst for alkene hydrosilation. M. Fasulo, M. C. Lipke and T. D. Tilley, Chem. Sci. 2013, 4, 3882. Water Oxidation Catalysis via Immobilization of the Dimanganese Complex [Mn2(μ- 383. O)2Cl(μ-O2CCH3)(bpy)2(H2O)](NO3)2 onto Silica. E. M. W. Rumberger, H. S. Ahn, A. T. Bell and T. D. Tilley, Dalton Trans. 2013, 42, 12238. The Osmium-Silicon Triple Bond: Synthesis and Reactivity of an Osmium Silylyne 382. Complex. P. G. Hayes, Z. Xu, C. Beddie, J. M. Keith, M. B. Hall and T. D. Tilley, J. Am. Chem. Soc. 2013, 135, 11780. Silane-Isocyanide Coupling Involving 1,1-Insertion of XylNC into the Si-H Bond of a σ- 381. Silane Ligand. M. C. Lipke and T. D. Tilley, J. Am. Chem. Soc. 2013, 135, 10298. A Remote Lewis Acid Trigger Dramatically Accelerates Biaryl Reductive Elimination from 380. a Platinum Complex. A. Liberman-Martin, R. G. Bergman and T.