S 1 Reaction Remember That a 3˚ Alkyl Halide Will Not Undergo a S 2 Reaction the Steric Hindrance in the Transition State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 + 3HC1 Effect of Water and Ammonia, Respectively, Upon
VOL. 23, 1937 CHEMISTRY: SLOB UTSKY, A UDRIETHAND CAMPBELL 611 ACID CATALYSIS IN LIQUID AMMONIA. I. AMMONOL YSIS OF DIETHYLMALONA TE BY CHARLES SLOBUTSEY, L. F. AUDRIETH AND R. W. CAMPBELL DEPARTMENT OF CHEMISTRY, UNIVERSITY OF ILLINOIS, URBANA, ILLINOIS Communicated November 3, 1937 Numerous investigations have shown that liquid ammonia possesses unusual properties as a solvent' for many inorganic and organic compounds. Like water it is the parent substance of a system of acids, bases and salts.2 Liquid ammonia also reacts directly with many substances. Solvolysis takes place and in the case of ammonia such reactions are termed ammono- lytic reactions. Just as phosphorus trichloride is hydrolyzed by water (1), so it may be ammonolyzed3 by ammonia to give the nitrogen analogs of phosphorous and hydrochloric acids (2). PCI + 3HOH -- P(OH)3 + 3HC1 (1) PCI3 + 5HNH2 -+ P(NH)(NH2) + 3NH4C1 (2) Many hydrolytic reactions are catalyzed in aqueous solutions by acids or bases. Thus, the inversion of cane sugar is catalyzed by acids and the velocity of the reaction is a function of the concentration of the hydrogen ion. Esters also undergo hydrolysis and such reactions are markedly catalyzed by the hydrogen ion, or in terms of the modern Bronsted concept of acidity, by the onium4 ion, or solvated proton. It was, therefore, to have been expected that the rate of ammonolysis of esters in liquid ammonia would be accelerated by the presence of ammonium salts, which have been shown to possess acid character in liquid ammonia. It has already been demonstrated qualitatively that ammonium salts do exert a catalytic effect in other ammonolytic reactions. -

Chapter 12 Lecture Notes
Chemical Kinetics “The study of speeds of reactions and the nanoscale pathways or rearrangements by which atoms and molecules are transformed to products” Chapter 13: Chemical Kinetics: Rates of Reactions © 2008 Brooks/Cole 1 © 2008 Brooks/Cole 2 Reaction Rate Reaction Rate Combustion of Fe(s) powder: © 2008 Brooks/Cole 3 © 2008 Brooks/Cole 4 Reaction Rate Reaction Rate + Change in [reactant] or [product] per unit time. Average rate of the Cv reaction can be calculated: Time, t [Cv+] Average rate + Cresol violet (Cv ; a dye) decomposes in NaOH(aq): (s) (mol / L) (mol L-1 s-1) 0.0 5.000 x 10-5 13.2 x 10-7 10.0 3.680 x 10-5 9.70 x 10-7 20.0 2.710 x 10-5 7.20 x 10-7 30.0 1.990 x 10-5 5.30 x 10-7 40.0 1.460 x 10-5 3.82 x 10-7 + - 50.0 1.078 x 10-5 Cv (aq) + OH (aq) → CvOH(aq) 2.85 x 10-7 60.0 0.793 x 10-5 -7 + + -5 1.82 x 10 change in concentration of Cv Δ [Cv ] 80.0 0.429 x 10 rate = = 0.99 x 10-7 elapsed time Δt 100.0 0.232 x 10-5 © 2008 Brooks/Cole 5 © 2008 Brooks/Cole 6 1 Reaction Rates and Stoichiometry Reaction Rates and Stoichiometry Cv+(aq) + OH-(aq) → CvOH(aq) For any general reaction: a A + b B c C + d D Stoichiometry: The overall rate of reaction is: Loss of 1 Cv+ → Gain of 1 CvOH Rate of Cv+ loss = Rate of CvOH gain 1 Δ[A] 1 Δ[B] 1 Δ[C] 1 Δ[D] Rate = − = − = + = + Another example: a Δt b Δt c Δt d Δt 2 N2O5(g) 4 NO2(g) + O2(g) Reactants decrease with time. -

Ochem ACS Review 23 Aryl Halides
ACS Review Aryl Halides 1. Which one of the following has the weakest carbon-chlorine bond? A. I B. II C. III D. IV 2. Which compound in each of the following pairs is the most reactive to the conditions indicated? A. I and III B. I and IV C. II and III D. II and IV 3. Which of the following reacts at the fastest rate with potassium methoxide (KOCH 3) in methanol? A. fluorobenzene B. 4-nitrofluorobenzene C. 2,4-dinitrofluorobenzene D. 2,4,6-trinitrofluorobenzene 4. Which of the following reacts at the fastest rate with potassium methoxide (KOCH 3) in methanol? A. fluorobenzene B. p-nitrofluorobenzene C. p-fluorotoluene D. p-bromofluorobenzene 5. Which of the following is the kinetic rate equation for the addition-elimination mechanism of nucleophilic aromatic substitution? A. rate = k[aryl halide] B. rate = k[nucleophile] C. rate = k[aryl halide][nucleophile] D. rate = k[aryl halide][nucleophile] 2 6. Which of the following is not a resonance form of the intermediate in the nucleophilic addition of hydroxide ion to para -fluoronitrobenzene? A. I B. II C. III D. IV 7. Which chlorine is most susceptible to nucleophilic substitution with NaOCH 3 in methanol? A. #1 B. #2 C. #1 and #2 are equally susceptible D. no substitution is possible 8. What is the product of the following reaction? A. N,N-dimethylaniline B. para -chloro-N,N-dimethylaniline C. phenyllithium (C 6H5Li) D. meta -chloro-N,N-dimethylaniline 9. Which one of the reagents readily reacts with bromobenzene without heating? A. -

Enzymatic Kinetic Isotope Effects from First-Principles Path Sampling
Article pubs.acs.org/JCTC Enzymatic Kinetic Isotope Effects from First-Principles Path Sampling Calculations Matthew J. Varga and Steven D. Schwartz* Department of Chemistry and Biochemistry, University of Arizona, Tucson, Arizona 85721, United States *S Supporting Information ABSTRACT: In this study, we develop and test a method to determine the rate of particle transfer and kinetic isotope effects in enzymatic reactions, specifically yeast alcohol dehydrogenase (YADH), from first-principles. Transition path sampling (TPS) and normal mode centroid dynamics (CMD) are used to simulate these enzymatic reactions without knowledge of their reaction coordinates and with the inclusion of quantum effects, such as zero-point energy and tunneling, on the transferring particle. Though previous studies have used TPS to calculate reaction rate constants in various model and real systems, it has not been applied to a system as large as YADH. The calculated primary H/D kinetic isotope effect agrees with previously reported experimental results, within experimental error. The kinetic isotope effects calculated with this method correspond to the kinetic isotope effect of the transfer event itself. The results reported here show that the kinetic isotope effects calculated from first-principles, purely for barrier passage, can be used to predict experimental kinetic isotope effects in enzymatic systems. ■ INTRODUCTION staging method from the Chandler group.10 Another method that uses an energy gap formalism like QCP is a hybrid Particle transfer plays a crucial role in a breadth of enzymatic ff reactions, including hydride, hydrogen atom, electron, and quantum-classical approach of Hammes-Schi er and co-work- 1 ers, which models the transferring particle nucleus as a proton-coupled electron transfer reactions. -

S.T.E.T.Women's College, Mannargudi Semester Iii Ii M
S.T.E.T.WOMEN’S COLLEGE, MANNARGUDI SEMESTER III II M.Sc., CHEMISTRY ORGANIC CHEMISTRY - II – P16CH31 UNIT I Aliphatic nucleophilic substitution – mechanisms – SN1, SN2, SNi – ion-pair in SN1 mechanisms – neighbouring group participation, non-classical carbocations – substitutions at allylic and vinylic carbons. Reactivity – effect of structure, nucleophile, leaving group and stereochemical factors – correlation of structure with reactivity – solvent effects – rearrangements involving carbocations – Wagner-Meerwein and dienone-phenol rearrangements. Aromatic nucleophilic substitutions – SN1, SNAr, Benzyne mechanism – reactivity orientation – Ullmann, Sandmeyer and Chichibabin reaction – rearrangements involving nucleophilic substitution – Stevens – Sommelet Hauser and von-Richter rearrangements. NUCLEOPHILIC SUBSTITUTION Mechanism of Aliphatic Nucleophilic Substitution. Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. For that reason, it attracts to nucleophile In organic chemistry and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which a leaving group(nucleophile) is replaced by an electron rich compound(nucleophile). The whole molecular entity of which the electrophile and the leaving group are part is usually called the substrate. The nucleophile essentially attempts to replace the leaving group as the primary substituent in the reaction itself, as a part of another molecule. The most general form of the reaction may be given as the following: Nuc: + R-LG → R-Nuc + LG: The electron pair (:) from the nucleophile(Nuc) attacks the substrate (R-LG) forming a new 1 bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. -

Alkyl Halides
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp3 orbital of an alkyl group. CHCl3 (Chloroform: organic solvent) CF2Cl2 (Freon-12: refrigerant CFC) CF3CHClBr (Halothane: anesthetic) Halogen atoms are more electronegative than carbon atoms, and so the C-Hal bond is polarized. The C-Hal (often written C-X) bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen. Ch06 Alkyl Halides (landscape).docx Page 1 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents. Ch06 Alkyl Halides (landscape).docx Page 2 Nomenclature According to IUPAC, alkyl halides are treated as alkanes with a halogen (Halo-) substituent. The halogen prefixes are Fluoro-, Chloro-, Bromo- and Iodo-. Examples: Often compounds of CH2X2 type are called methylene halides. (CH2Cl2 is methylene chloride). CHX3 type compounds are called haloforms. (CHI3 is iodoform). CX4 type compounds are called carbon tetrahalides. (CF4 is carbon tetrafluoride). Alkyl halides can be primary (1°), secondary (2°) or tertiary (3°). Other types: A geminal (gem) dihalide has two halogens on the same carbon. -
Microtest III Print File
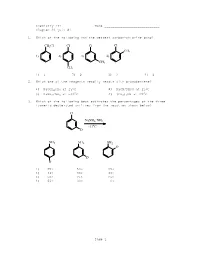
Chemistry 211 Name ____________________________ Chapter 23 Quiz #1 1. Which of the following has the weakest carbon-chlorine bond? CH2Cl Cl Cl Cl CH3 1) 2) 3) 4) CH3 CH3 1) 1 2) 2 3) 3 4) 4 2. Which one of the reagents readily reacts with bromobenzene? 1) NaOCH2CH3 at 250C 2) NaCN/DMSO at 250C 3) NaNH2/NH3 at -330C 4) (CH3)2NH at 250C 3. Which of the following best estimates the percentages of the three isomeric deuterated anilines from the reaction shown below? Cl NaNH2, NH3 -33oC D NH 2 NH 2 NH 2 D D D 1) 25% 50% 25% 2) 33% 33% 33% 3) 50% 25% 25% 4) 66% 33% 0% Page 1 4. Which of the following is (are) true concerning the intermediate in the addition-elimination mechanism of the reaction below? F OCH3 NaOCH 3 intermediate NO2 NO2 A. The intermediate is aromatic. B. The intermediate is a resonance stabilized anion. C. Electron withdrawing groups on the benzene ring stabilize the intermediate. 1) only A 2) only B 3) A and C 4) B and C 5. Carbon-14 labelled chlorobenzene is reacted with sodium amide in ammonia as shown below. Which of the following depicts the carbon-14 label in the product(s)? Cl NaNH2/NH3 -33oC Cl Cl Cl 1) 3) and (about 50% of each) Cl Cl Cl 2) 4) and (about 50% of each) 1) 1 2) 2 3) 3 4) 4 6. Which of the following reacts at the fastest rate with potassium methoxide (KOCH3) in methanol? 1) fluorobenzene 2) 4-nitrofluorobenzene 3) 2,4-dinitrofluorobenzene 4) 2,4,6-trinitrofluorobenzene Page 2 7. -

Solvolysis Reaction Kinetics, Rates and Mechanism for Phenyl N-Phenyl Phosphoramidochloridate
Studies of Phenyl N-Phenyl Phosphoramidochloridate Bull. Korean Chem. Soc. 2014, Vol. 35, No. 8 2465 http://dx.doi.org/10.5012/bkcs.2014.35.8.2465 Solvolysis Reaction Kinetics, Rates and Mechanism for Phenyl N-Phenyl Phosphoramidochloridate Hojune Choi, Kiyull Yang, Han Joong Koh,†,* and In Sun Koo* Department of Chemistry Education and Research Instituted of Natural Science, Gyeongsang National University, Jinju 660-701, Korea. *E-mail: [email protected] †Department of Science Education, Chonju National University of Education, Chonju, Korea. *E-mail: [email protected] Received March 29, 2014, Accepted April 29, 2014 The rate constants of solvolysis of phenyl N-phenyl phosphoramidochloridate (PhNHPO(Cl)OPh, Target Compound-TC1) have been determined by a conductivity method. The solvolysis rate constants of TC1 are well correlated with the extended Grunwald-Winstein equation, using the NT solvent nucleophilicity scale and YCl solvent ionizing scale, and sensitivity values of 0.85 ± 0.14 and 0.53 ± 0.04 for l and m, respectively. These l and m values were similar to those obtained previously for the complex chemical substances dimethyl thiophosphorochloridate; N,N,N',N'-tetramethyldiamidophosphorochloridate; 2-phenyl-2-ketoethyl tosylate; diphenyl thiophosphinyl chloride; and 9-fluorenyl chloroformate. As with the five previously studied solvolyses, an SN2 pathway is proposed for the solvolyses of TC1. For four representative solvents, the rate constants were measured at several temperatures, and activation parameters (∆H≠ and ∆S≠) were estimated. These activation parameters are also in line with the values expected for an SN2 reaction. Key Words : Phenyl N-phenyl phosphoramidochloridate, Extended Grunwald-Winstein equation, SN2 mech- anism, Activation parameters Introduction mediate or is the result of a single transition state (TS).2 The reaction mechanism of phosphoryl compounds, such Phosphoryl transfer is an important aspect of biological as that of ‘phenyl N-phenyl phosphoramidochloridate’ chemistry and organic synthesis. -

Enthalpy and Free Energy of Reaction
KINETICS AND THERMODYNAMICS OF REACTIONS Key words: Enthalpy, entropy, Gibbs free energy, heat of reaction, energy of activation, rate of reaction, rate law, energy profiles, order and molecularity, catalysts INTRODUCTION This module offers a very preliminary detail of basic thermodynamics. Emphasis is given on the commonly used terms such as free energy of a reaction, rate of a reaction, multi-step reactions, intermediates, transition states etc., FIRST LAW OF THERMODYNAMICS. o Law of conservation of energy. States that, • Energy can be neither created nor destroyed. • Total energy of the universe remains constant. • Energy can be converted from one form to another form. eg. Combustion of octane (petrol). 2 C8H18(l) + 25 O2(g) CO2(g) + 18 H2O(g) Conversion of potential energy into thermal energy. → 16 + 10.86 KJ/mol . ESSENTIAL FACTORS FOR REACTION. For a reaction to progress. • The equilibrium must favor the products- • Thermodynamics(energy difference between reactant and product) should be favorable • Reaction rate must be fast enough to notice product formation in a reasonable period. • Kinetics( rate of reaction) ESSENTIAL TERMS OF THERMODYNAMICS. Thermodynamics. Predicts whether the reaction is thermally favorable. The energy difference between the final products and reactants are taken as the guiding principle. The equilibrium will be in favor of products when the product energy is lower. Molecule with lowered energy posses enhanced stability. Essential terms o Free energy change (∆G) – Overall free energy difference between the reactant and the product o Enthalpy (∆H) – Heat content of a system under a given pressure. o Entropy (∆S) – The energy of disorderness, not available for work in a thermodynamic process of a system. -

Initial State and Transition State Solvation for the Solvolysis of ^Razis
宙시 State and Transition State Solvation for the Solvolysis of trans-[Co(N-ete^2ClJ^ Bull. Korean Chem. Soc.f Vol. 11, No. 4, 1990 309 aqueous HC1 solution. The reaction product was extracted 2, (a) H. Kotsuki, Y. Ushio, I. Kadota, and M. O산 ii,/ ()rg. three times with ether (20 ml x 3) and the organic옹 were Chem., 54, 5153 (1989); (b) H. Kots니 d, Y. Ushio, I. separated and dried over anhydrous magnesium sulfate. The Kadota, and M. Ochi, Chem. Lett., 927 (1988); (c) B. P. ether was removed by rotatory evaporator. The GLC ana M니 ndy and M. Bjorklund. Tetrahedron Lett., 26, 3899 lysis indicated an exo/endo ratio of 83:17 (quantitative yield). (1985); (d) H. A. Bates and P.-N. Deng, / ()rg. Chem., General procedure for endo-5,7-dimethyl-6,8-dioxabicy- 48, 4479 (1983). clo[3.2.1]octane (5). To a dried ZnCl2(2 eq.) was added drop 3, (a) B. P. Mundy and W. G. Bommann,/ ()rg. Chem., 49, wise 0.2 g (1.4 mmol) of methyl vinyl keton dimer (1) in 2 ml 5264 (1984); (b) T. Cohen and M. Bhupathy, Tetrahed- ) of CH2C12 and 3 eq. of Zn(BH4 2 (0.8M solution in THF) res ron Lett., 24, 4163 (1983); (c) P. Chaquin, J-P. Morizur, pectively at 0°C. After 2 hrs stirring at 0°C, 10 mZ of 15% and J. KossanyiJ. Am. Chem. Sog, 99,903(1977); (d) K. aqueous HC1 solution was added to this reaction mixture and Mori, Tetrahedron, 30, 4223 (1974). -

Kinetics and Mechanistic Investigation of Epoxide/CO2 Cycloaddition by a Synergistic Catalytic Effect of Pyrrolidinopyridinium Iodide and Zinc Halides
Kinetics and mechanistic investigation of epoxide/CO2 cycloaddition by a synergistic catalytic effect of pyrrolidinopyridinium iodide and zinc halides Abdul Rehman a, b,*, Valentine C. Eze a, M.F.M. Gunam Resula, Adam Harveya a School of Engineering, Merz Court, Newcastle University, Newcastle Upon Tyne, NE1 7RU, UK b Department of Chemical and Polymer Engineering, University of Engineering and Technology Lahore, Faisalabad Campus, Pakistan *[email protected] Abstract Formation of styrene carbonate (SC) by the cycloaddition of CO2 to styrene oxide (SO) catalysed by pyrrolidinopyridinium iodide (PPI) in combination with zinc halides (ZnCl2, ZnBr2 and ZnI2) was investigated. Complete conversion of the SO to SC was achieved in 3 h with 100% selectivity using 1:0.5 molar (PPI/ZnI2) catalyst ratio under mild reaction conditions i.e. 100 °C and 10 bar CO2 pressure. The synergistic effect of ZnI2 and PPI resulted in more than 7-fold increase in reaction rate than using PPI alone. The cycloaddition reaction demonstrated the first-order dependence with respect to the epoxide, CO2 and catalyst concentrations. Moreover, the kinetic and thermodynamic activation parameters of SC formation were determined using the Arrhenius and Eyring equations. The positive values of ΔH‡ (42.8 kJ mol–1) and ΔG‡ (102.3 kJ mol–1) revealed endergonic and chemically controlled nature of the reaction, whereas the large negative values of ΔS‡ (–159.4 J mol–1 K–1) indicate a highly ordered activated complex at the transition state. The activation energy for SC formation catalyzed by PPI alone was found to be 73.2 kJ mol–1 over a temperature range of 100–140 °C, –1 which was reduced to 46.1 kJ mol when using PPI in combination with ZnI2 as a binary catalyst. -

Chemical Kinetics, but Were Afraid to Ask…
Everything You Always Wanted to Know (or better yet, need to know) About Chemical Kinetics, But Were Afraid to Ask… Overall Reactions An overall chemical reaction indicates reactants and products in the appropriate stoichiometric quantities. Overall chemical reactions can be used to predict quantities consumed or produced and write equilibrium expressions. Consequently, overall reactions are used in thermodynamic calculations, such as energy changes and equilibrium concentrations. However, the overall reaction provides no information about the molecular process involved in transforming reactants into products, the nature and number of individual steps involved, the presence of intermediates or the role of catalysis. Consequently, overall reactions provide no information about how fast a reaction proceeds or the various factors that influence reaction rates. For example, the overall reaction for the combustion of methane is given by; CH4(g) + O2(g) ==== CO2(g) + 2 H2O(g) From which we can write and equilibrium expression, calculate free energy changes and so on, but which provide little insight into how long it may take for the reaction to occur or what species might act as catalysts. Reaction Mechanisms Mechanisms provide the individual steps that occur on the molecular level in transforming reactants into products. (Whereas the overall reaction indicates where we start and end up, the mechanism tells us how we got there). Reaction mechanisms are composed of a series of elementary processes (steps) which are indicative of the molecular encounters. Because of this, mechanisms are intimately related to chemical kinetics. For example, the first two steps in the combustion of methane are given by; CH4 + OH Æ CH3 + H2O slow CH3 + O2 Æ CH3O2 fast Interestingly, mechanisms are never proven but rather postulated and checked for consistency with observed experimental kinetic data.