AX Pharmaceutical Corp Product List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Management of Common Skin Conditions in General Practice
Management of Common Skin Conditions In General Practice including the “red rash made easy” © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care University of Auckland, Tamaki Campus Reviewed by Hon A/Prof Amanda Oakley - 2019 http://www.dermnetnz.org Management of Common Skin Conditions In General Practice Contents Page Derm Map 3 Classic location: infants & children 4 Classic location: adults 5 Dermatology terminology 6 Common red rashes 7 Other common skin conditions 12 Common viral infections 14 Common bacterial infections 16 Common fungal infections 17 Arthropods 19 Eczema/dermatitis 20 Benign skin lesions 23 Skin cancers 26 Emergency dermatology 28 Clinical diagnosis of melanoma 31 Principles of diagnosis and treatment 32 Principles of treatment of eczema 33 Treatment sequence for psoriasis 34 Topical corticosteroids 35 Combination topical steroid + antimicrobial 36 Safety with topical corticosteroids 36 Emollients 37 Antipruritics 38 For further information, refer to: http://www.dermnetnz.org And http://www.derm-master.com 2 © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care, University of Auckland, Tamaki Campus. Management of Common Skin Conditions In General Practice DERM MAP Start Is the patient sick ? Yes Rash could be an infection or a drug eruption? No Insect Bites – Crop of grouped papules with a central blister or scab. Is the patient in pain or the rash Yes Infection: cellulitis / erysipelas, impetigo, boil is swelling, oozing or crusting? / folliculitis, herpes simplex / zoster. Urticaria – Smooth skin surface with weals that evolve in minutes to hours. No Is the rash in a classic location? Yes See our classic location chart . -

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Treatment of Diseases and Conditions Mediated By
(19) TZZ_ ___T (11) EP 1 572 115 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 38/07 (2006.01) A61K 38/08 (2006.01) 21.01.2015 Bulletin 2015/04 A61K 38/17 (2006.01) A61K 38/38 (2006.01) (21) Application number: 03799853.1 (86) International application number: PCT/US2003/037901 (22) Date of filing: 25.11.2003 (87) International publication number: WO 2004/050023 (17.06.2004 Gazette 2004/25) (54) TREATMENT OF DISEASES AND CONDITIONS MEDIATED BY INCREASED PHOSPHORYLATION BEHANDLUNG VON ERKRANKUNGEN UND ZUSTÄNDEN,DIE DURCH ERHÖHTE PHOSPHORYLIERUNG VERMITTELT WERDEN TRAITEMENT DE MALADIES ET D’ETATS A MEDIATION DE PHOSPHORYLATION ACCRUE (84) Designated Contracting States: • JIANG B ET AL: "Phosphopeptides derived from AT BE BG CH CY CZ DE DK EE ES FI FR GB GR hen egg yolk phosvitin: effect of molecular size HU IE IT LI LU MC NL PT RO SE SI SK TR on the calcium-binding properties." BIOSCIENCE, BIOTECHNOLOGY, AND (30) Priority: 27.11.2002 US 429924 P BIOCHEMISTRY MAY 2001, vol. 65, no. 5, May 2001 (2001-05), pages 1187-1190, XP002549556 (43) Date of publication of application: ISSN: 0916-8451 14.09.2005 Bulletin 2005/37 • KATAYAMA SHIGERU ET AL: "Antioxidative stress activity of oligophosphopeptides derived (73) Proprietor: Ampio Pharmaceuticals, Inc. from hen egg yolk phosvitin in Caco-2 cells." Englewood, CO 80112 (US) JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 8 FEB 2006, vol. 54, no. 3, 8 February (72) Inventor: BAR-OR, David 2006 (2006-02-08), pages 773-778, XP002549557 Englewood, CO 80110 (US) ISSN: 0021-8561 • OKAMOTO ET AL: ’The interleukin-8 AP-1 and (74) Representative: Weber, Joachim kappa B-like sites are genetic end targets of Hoefer & Partner FK506-sensitive pathway accompanied by Patentanwälte calcium mobilization.’ JOURNAL OF Pilgersheimer Strasse 20 BIOLOGICAL CHEMISTRY vol. -

WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T (51) International Patent Classification: (74) Agents: CHOTIA, Meenakshi et al; K&S Partners | Intel A 25/12 (2006.01) A61K 8/11 (2006.01) lectual Property Attorneys, 4121/B, 6th Cross, 19A Main, A 25/34 (2006.01) A61K 8/49 (2006.01) HAL II Stage (Extension), Bangalore 560038 (IN). A01N 37/06 (2006.01) A61Q 5/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A O 43/12 (2006.01) A61K 31/44 (2006.01) kind of national protection available): AE, AG, AL, AM, AO 43/40 (2006.01) A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A01N 57/12 (2006.01) A61K 9/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, AOm 59/16 (2006.01) A61K 31/496 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/IB20 14/06 1925 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 3 June 2014 (03.06.2014) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Chelating Drug Therapy: an Update
Open Access Austin Journal of Genetics and Genomic Research Review Article Chelating Drug Therapy: An Update Vijay Kumar1, Ashok Kumar2*, Sandeep Kumar Singh1, Manoj Kumar3, Surendra Kumar2, Dinesh Abstract 4 5 Kumar and Ragni Singh Purpose: To study the clinical effects of metal toxicity and current 1Department of Neurology, SGPGIMS, India recommendations for management, including chelation therapy, are reviewed. 2Department of Medical Genetics, SGPGIMS, India 3Department of Microbiology, SGPGIMS, India Summary: Metals are essential to many biological processes, but excess 4Department of Chemistry, Dr. R.M.L. Avadh University, of it becomes hazardous to life. These are necessary for cell growth, electron India transport chain, several enzymatic activities and response of immune systems. 5Bheem Rao Ambedkar Bihar University, India They also serve as a cofactor for several enzymes. Chelation therapy is used for clinical management of the excess of metal. However, each metal requires *Corresponding author: Ashok Kumar, Department a specific chelation agent. A chelate is a compound form between metal and a of Medical Genetics, Sanjay Gandhi Post Graduate compound that contains two or more potential ligands. A promising Fe chelator Institute of Medical Sciences, Lucknow, India is Desferrioxamine (Desferal). Penicillamine and Trientine are uses for copper Received: March 12, 2015; Accepted: April 24, 2015; chelation. Meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto- Published: April 27, 2015 Propanesulphonate (DMPS) can be used as effective chelator of mercury. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead. Conclusion: Metal toxicity remains a significant public health concern. Elimination of elevated metal ions can be achieved by proper chelation agents. -

Changes in Tissue Gadolinium Biodistribution Measured in an Animal Model Exposed to Four Chelating Agents
Japanese Journal of Radiology (2019) 37:458–465 https://doi.org/10.1007/s11604-019-00835-1 ORIGINAL ARTICLE Changes in tissue gadolinium biodistribution measured in an animal model exposed to four chelating agents Türker Acar1,6 · Egemen Kaya2 · Mustafa Deniz Yoruk3 · Neslihan Duzenli4 · Recep Selim Senturk4 · Cenk Can4 · Lokman Ozturk3 · Canberk Tomruk5 · Yigit Uyanikgil5 · Frank J. Rybicki6 Received: 17 December 2018 / Accepted: 22 March 2019 / Published online: 30 March 2019 © Japan Radiological Society 2019 Abstract Purpose This study investigated the potential to reduce gadolinium levels in rodents after repetitive IV Gadodiamide admin- istration using several chelating agents. Materials and methods The following six groups of rats were studied. Group 1: Control; Group 2: Gadodiamide only; Group 3: Meso-2,3-Dimercaptosuccinic acid (DMSA) + Gadodiamide; Group 4: N-Acetyl-L-cysteine (NAC) + Gadodiamide; Group 5: Coriandrum sativum extract + Gadodiamide; and Group 6: Deferoxamine + Gadodiamide. Brain, kidney, and blood samples were evaluated via inductively coupled plasma mass spectrometry. The brain was also evaluated histologically. Results Kidney gadolinium levels in Groups 4 and 5 were approximately double that of Group 2 (p = 0.033 for each). There was almost no calcifcation in rat hippocampus for Group 4 rodents when compared with Groups 2, 3, 5 and 6. Conclusion Our preliminary study shows that excretion to the kidney has a higher propensity in NAC and Coriandrum sati- vum groups. It may be possible to change the distribution of gadolinium by administrating several agents. NAC may lower Gadodiamide-induced mineralization in rat hippocampus. Keywords Gadolinium deposition · Dimercaptosuccinic acid · N-Acetyl-L-cysteine · Coriandrum sativum · Deferoxamine Introduction However, the safety profle of gadolinium-based agents [1] was questioned after the discovery of nephrogenic sys- Gadolinium-based contrast agents (GBCA) have been safely temic fbrosis [2], a scleroderma-like disease characterized used in diagnostic radiology since the 1980s. -
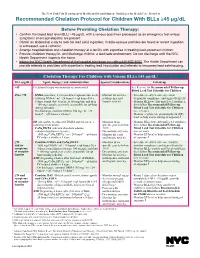
Recommended Chelation Protocol for Children with Blls ≥45 Μg/Dl
The New York City Department of Health and Mental Hygiene Guidelines for Health Care Providers Recommended Chelation Protocol for Children With BLLs ≥45 μg/dL Before Providing Chelation Therapy: • Confirm the blood lead level (BLL) ≥45 μg/dL with a venous specimen processed as an emergency test unless symptoms of encephalopathy are present. • Obtain an abdominal x-ray to look for lead solid ingestion; if radio-opaque particles are found or recent ingestion is witnessed, use a cathartic. • Arrange hospitalization and chelation therapy at a facility with expertise in treating lead-poisoned children. • Provide chelation therapy in, and discharge child to, a lead-safe environment. Do not discharge until the NYC Health Department inspects the home. • Inform the NYC Health Department of the hospital admission by calling 646-632-6002. The Health Department can provide referrals to providers with expertise in treating lead intoxication and referrals to temporary lead-safe housing. Chelation Therapy For Children with Venous BLLs ≥45 μg/dL1 BLL (μg/dL) Agent, Dosage,* and Administration Special Considerations Follow-up <45 Chelation therapy not routinely recommended See Reverse for Recommended Follow-up Blood Lead Test Schedule for Children 45 to <70 • DMSA (succimer, 2,3-meso-dimercaptosuccinic acid) • Monitor for anemia, • Schedule weekly health care visits • 1050 mg DMSA / m2 / 24 hours* ÷ q8 hours PO x neutropenia, and to monitor compliance and signs of toxicity. 5 days; round dose to nearest 100 mg/day, and then hepatic toxicity. • Monitor BLLs weekly until level stabilizes, ÷ 100-mg capsules as evenly as possible for q8-hour then follow Recommended Follow-up dosing schedule. -

Taking a Dermatological Approach to Treating Ocular Surface Diseases
Taking a Dermatological Approach to treating Ocular Surface Diseases MARC GLEESON | CHIEF EXECUTIVE OFFICER OIS@SECO 2020 for Meibomian Gland Dysfunction, Contact Lens Discomfort & Blepharitis Copyright© Azura Ophthalmics | Confidential Meibomian Gland Contact Lens Blepharitis Demodex Dysfunction Discomfort Patient Administered Patient Administered Patient Administered Rx Proven activity against Rx Chronic Treatment Rx Chronic Treatment Treatment applied to Demodex applied to the eyelid applied to the eyelid the eyelid Allows continuation of New Chemical Entity In Office Physician Only Contact Lens Wear Administered Rx Treatment Lid Margin Involvement Hyperkeratinization of the Gland Orifice +/- Inflammation Copyright© Azura Ophthalmics | Confidential (MGD) Reduced secretion of lipids leads to instability of the tear film Modified sebaceous (oil-producing) and drying of the ocular surface, leading to damage and the glands responsible for secreting the signs and symptoms of dry eye disease (DED). outer lipid layer (meibum) of the tear film, which lubricates the ocular Obstructive MGD is the most common cause of evaporative surface during blinking and protects dry DED,1,3 and clinical signs of obstructive MGD are present against tear evaporation1,2 in 86% of DED patients4 MGD traditionally regarded as a hypersecretory disorder associated with bacterial infection and inflammation,1 which has guided the approach to treatment, often unsuccessfully 1Blackie et al. Cornea. 2010 | 2Knop et al. Invest Ophthalmol Vis Sci. 2011 | 3Baudouin et al. -

Effect of Chromium(VI) on Serum Iron and Removal of Its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats
American Journal of Pharmacology and Toxicology 8 (4): 164-169, 2013 ISSN: 1557-4962 ©2013 Science Publication doi:10.3844/ajptsp.2013.164.169 Published Online 8 (4) 2013 (http://www.thescipub.com/ajpt.toc) Effect of Chromium(VI) on Serum Iron and Removal of its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats 1S. Jamil A. Fatemi, 1Marzieh Iranmanesh and 2Faezeh Dahooee Balooch 1Department of Chemistry, Faculty of Sciences, Islamic Azad University, Kerman Branch, Kerman, Iran 2Department of Chemistry, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran Received 2013-09-26, Revised 2013-10-21; Accepted 2013-10-30 ABSTRACT The present research is aimed to characterize the potential efficiency of two chelators after chromium(VI) administration for 60 days following two doses of 15 and 30 mg kg −1 chromium(VI) per body weight daily to male rats. However, the hypothesis that the two chelators might be more efficient as combined therapy than as single therapy in removing chromium(VI) from bood serum was considered. In this way, two known chelators deferasirox and deferiprone were chosen and tested in the acute rat model. Two chelators were given orally as a single or combined therapy for a period of one week. Chromium(VI) and iron concentrations in blood were determined by flame atomic absorption spectroscopy method. Chromium is one of the most widely used industrial metals. Several million workers worldwide are estimated to be exposed to chromium compounds in an array of industries. Chromium(VI) is more readily absorbed by both inhalation and oral routes. Ingestion of large amounts of chromium(VI) can lead to severe respiratory, cardiovascular, gastrointestinal, hepatic and renal damage and potentially death. -

The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island Poison Potential Antidote Acetaminophen n-Acetylcysteine [Mucomyst®] Anticholinergics Physostigmine [Antilirium®] Benzodiazepines Flumazenil [Romazicon®] Beta-adrenergic blockers Glucagon Botulinum toxin Trivalent ABE botulinum antitoxin Calcium chloride or calcium gluconate Calcium channel blockers Hyperinsulinemia-euglycemia (HIE) therapy Atropine Carbamates Pralidoxime (2-PAM) [Protopam®] Carbon monoxide Oxygen; Hyperbaric oxygen (HBO) Clonidine Naloxone [Narcan®] Cyanide Cyanide Kit (Amyl/sodium nitrite, sodium thiosulfate) Digoxin (Cardiac glycosides) Digoxin Immune FAB Ovine [Digibind®, Digifab®] Epi Pen (Epinephrine SQ) Phentolamine [Regitine®] Ethanol Ethylene glycol 4-Methylpyrazole (Fomepizole) [Antizol®] Fluoride Calcium chloride or calcium gluconate Heparins Protamine Hydrofluoric acid Calcium chloride or calcium gluconate Hydrogen sulfide Oxygen; Hyperbaric oxygen (HBO); Sodium nitrite Iodine Starch Isoniazid Pyridoxine (Vitamin B6 ) METALS Dimercaprol [BAL] Arsenic Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Bismuth Dimercaprol [BAL] Copper D-Penicillamine [Cuprimine®] Gold Dimercaprol [BAL] Iron Deferoxamine [Desferal®] Dimercaprol [BAL] Edetate calcium disodium (Calcium EDTA) Lead Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] D-Penicillamine [Cuprimine®] Dimercaprol [BAL] Mercury Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Ethanol Methanol 4-Methylpyrazole (Fomepizole) [Antizol®] Methemoglobinemic agents Methylene -

DRAFT NOTE for GUIDANCE on ORGANIC IMPURITIES in 3 ACTIVE PHARMACEUTICAL INGREDIENTS and FINISHED 4 PHARMACEUTICAL PRODUCTS 5 (July 2016)
Working document QAS/15.606/Rev2 July 2016 Draft document for comment 1 2 DRAFT NOTE FOR GUIDANCE ON ORGANIC IMPURITIES IN 3 ACTIVE PHARMACEUTICAL INGREDIENTS AND FINISHED 4 PHARMACEUTICAL PRODUCTS 5 (July 2016) 6 7 DRAFT FOR COMMENT 8 Should you have any comments on the attached text, please send these to Dr Herbert Schmidt, Medicines Quality Assurance, Technologies, Standards and Norms, World Health Organization, 1211 Geneva 27, Switzerland; email: [email protected]; fax: (+41 22) 791 4730) by 16 September 2016. In order to speed up the process for receiving draft monographs and for sending comments, please let us have your email address (to [email protected]) and we will add it to our electronic mailing list. Please specify if you wish to receive monographs. 9 10 ____________________________________________________________________________________________________ 11 © World Health Organization 2016 12 All rights reserved. 13 This draft is intended for a restricted audience only, i.e. the individuals and organizations having received this draft. The 14 draft may not be reviewed, abstracted, quoted, reproduced, transmitted, distributed, translated or adapted, in part or in whole, 15 in any form or by any means outside these individuals and organizations (including the organizations' concerned staff and 16 member organizations) without the permission of the World Health Organization. The draft should not be displayed on any 17 website. 18 Please send any request for permission to: 19 Dr Sabine Kopp, Group Lead, Medicines Quality Assurance, Technologies, Standards and Norms, Department of Essential 20 Medicines and Health Products, World Health Organization, CH-1211 Geneva 27, Switzerland. 21 Fax: (41-22) 791 4730; email: [email protected].