Specialty Guideline Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -
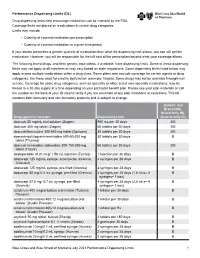
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage -

ART Drugs Page: 1 of 8
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.02 Section: Prescription Drugs Effective Date: July 1, 2021 Subsection: Endocrine and Metabolic Drugs Original Policy Date: January 1, 2011 Subject: ART Drugs Page: 1 of 8 Last Review Date: June 17, 2021 ART Drugs Description Bravelle (urofollitropin) Cetrotide (cetrorelix) Clomid, Clomiphene Powder, Serophene (clomiphene citrate) Crinone, Endometrin, Progesterone in Oil, Progesterone Powder, Prometrium (progesterone) Follistim AQ (follitropin beta) Gonal-F, Gonal F RFF (follitropin alfa) Ganirelix (ganirelix) Menopur (menotropins) Milprosa (progesterone) Background Assisted Reproductive Technologies (ART) represent a group of non-coital manipulations and processes that manipulate ova and/or sperm to achieve a pregnancy. The most well-known examples are ovulation induction, intrauterine insemination and in-vitro fertilization. ART and infertility drugs used in conjunction with ART procedures or erectile or sexual dysfunction, weight loss, performance enhancement and anti-aging are not covered benefits. The diagnosis of hypogonadotropic hypogonadism is an off-label indication for these medications. A variety of drugs are used to manipulate the hypothalamic-pituitary-gonadal axis in order to induce ovulation in females known as controlled ovarian hyperstimulation (COH). Some of these pharmacologic agents are used for additional clinical care indications. Drugs Included in Infertility Drugs / ART Criteria • Antagon (ganirelix) – inhibition -

Appendiks Til Marte Myhre Reigstad, Inger Kristin Larsen, Ritsa Storeng
Appendiks til Marte Myhre Reigstad, Inger Kristin Larsen, Ritsa Storeng. Kreftrisiko hos mor og barn etter fertilitetsbehandling. Tidsskr Nor Legeforen 2018; 138. doi: 10.4045/tidsskr.17.1098. Dette appendikset er et tillegg til artikkelen og er ikke bearbeidet redaksjonelt. Ramme 1 Beskrivelse av søkestrengen Søket består av 3 deler; fertilitetsbehandling, risiko, kreft som er kombinert med AND. Det er søkt med kontrollerte emneord (MeSH eller EMTREE), ord i tittel/abstakt /forfatters nøkkelord, kombinert med OR, innenfor de tre delene. EMBASE: artificial insemination/ or embryo disposition/ or exp embryo transfer/ or fertilization in vitro/ or in vitro oocyte maturation/ or intracytoplasmic sperm injection/ or ovulation induction/ or in vitro oocyte maturation/ or intracytoplasmic sperm injection/ or ovulation induction/ or superovulation/ or chorionic gonadotropin/ or clomifene/ or clomifene citrate/ or corifollitropin alfa/ or follitropin/ or gonadotropin/ or human menopausal gonadotropin/ or recombinant chorionic gonadotropin/ or recombinant follitropin/ or recombinant follitropin plus recombinant luteinizing hormone/ or recombinant luteinizing hormone/ or urofollitropin/ or gonadorelin/ or (gonadotropin* or clomifene or clomiphene or corifollitropin* or follitropin* or recombinant luteinizing hormone or urofollitropin* or gonadorelin* or buserelin* or leuprolide or menotropin* or nafarelin* or (fertilization* adj3 vitro) or (fertilisation* adj3 vitro) or (fertilization* adj3 invitro) or (fertilisation* adj3 invitro) or ivf or intracytoplasmic -

Pharmacy Prior Authorization Guideline
Harvard Pilgrim Health Care – Pharmacy Prior Authorization Guideline Guideline Name Gonadotropins and Antigonadotropins: Bravelle (urofollitropin), Cetrotide (cetrorelix), chorionic gonadotropin, Ganirelix, Gonal-F (follitropin alfa), Follistim AQ (follitropin beta), Menopur (menotropin), Novarel (chorionic gonadotropin), Ovidrel (choriogonadotropin alfa), and Pregnyl (chorionic gonadotropin) 1 . Criteria Product Name: Bravelle, Cetrotide, generic chorionic gonadotropin, Ganirelix, Gonal-F, Gonal- F RFF, Menopur, Novarel, Ovidrel, Pregnyl Diagnosis Gonadotropin therapy for females with infertility** Approval Length As requested up to 7 Month(s)* Guideline Type Prior Authorization Approval Criteria 1 - Patient has been approved for infertility services through a Harvard Pilgrim Health Care (HPHC) medical authorization^ Notes ^ The approval duration for formulary infertility medications (authorized by HPHC Pharmacy Benefit) will be approved 1 month prior to the date of the medical infertility services authorization (authorized by HPHC Medical Benefit) plus an additional 6 months unless specified otherwise on PA request (for a total of up to 7 months). *For approvals: Please approve at GPI List Name HPHCMEDIVF. **Some plans EXCLUDE gonadotropin products for infertility and claims will reject as Plan Exclusion: Plan excludes meds for infertility. Product Name: Follistim AQ Diagnosis Gonadotropin therapy for females with infertility** Approval Length As requested up to 7 Month(s) Guideline Type Non-Formulary Approval Criteria 1 - Patient has -

ART Drugs Page: 1 of 6
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.02 Section: Prescription Drugs Effective Date: January 1, 2020 Subsection: Endocrine and Metabolic Drugs Original Policy Date: January 1, 2011 Subject: ART Drugs Page: 1 of 6 Last Review Date: December 6, 2019 ART Drugs Description Bravelle (urofollitropin), Cetrotide (cetrorelix), Clomid, Clomiphene Powder, Serophene (clomiphene citrate), Crinone, Endometrin, Progesterone in Oil, Progesterone Powder, Prometrium (progesterone), Follistim AQ (follitropin beta), Gonal-F, Gonal F RFF (follitropin alfa), Ganirelix (ganirelix), Eligard, Lupron Depot (leuprolide), Menopur (menotropins) Background Assisted Reproductive Technologies (ART) represent a group of non-coital manipulations and processes that manipulate ova and/or sperm to achieve a pregnancy. The most well-known examples are ovulation induction, intrauterine insemination and in-vitro fertilization. ART and infertility drugs used in conjunction with ART procedures or erectile or sexual dysfunction, weight loss, performance enhancement and anti-aging are not covered benefits. The diagnosis of hypogonadotropic hypogonadism is an off label indication for these medications. A variety of drugs are used to manipulate the hypothalamic-pituitary-gonadal axis in order to induce ovulation in females known as controlled ovarian hyperstimulation (COH). Some of these pharmacologic agents are used for additional clinical care indications. Drugs Included in Infertility Drugs / ART Criteria Antagon -

Standard Specialty PA and QL List January 2015
Standard Specialty PA and QL List January 2015 Standard PA or PA with QL Programs Therapeutic Category Drug Name Quantity Limit Anti-infectives Antiretrovirals, Hepatitis B BARACLUDE (entecavir) 1 tab/day BARACLUDE (entecavir) Soln 630 ml/30days HEPSERA (adefovir) 1 tab/day TYZEKA (telbivudine ) 1 tab/day Antiretrovirals, HIV FUZEON (enfuvirtide) 60 vials or 1 kit/30 days SELZENTRY (maraviroc) None TRUVADA (emtricitabine/tenofovir) None Cardiology Antilipemic JUXTAPID (lomitapide) 20 mg 3 tabs/day JUXTAPID (lomitapide) 5 mg, 10 mg 1 tab/day KYNAMRO (mipomersen) 4 syringes/28 days Pulmonary Arterial Hypertension ADCIRCA (tadalafil) 2 tabs/day ADEMPAS (riociguat) 90 tabs/30 days FLOLAN (epoprostenol) None LETAIRIS (ambrisentan) 1 tab/day OPSUMIT (macitentan) 1 tab/day ORENITRAM (treprostinil diolamine) None REMODULIN (treprostinil) None REVATIO (sildenafil) 3 tabs or vials/day TRACLEER (bosentan) 2 tabs/day TYVASO (treprostinil) 1 ampule/day VELETRI (epoprostenol) None VENTAVIS (iloprost) 9 ampules/day Vasopressors NORTHERA (droxidopa) None Central Nervous System Anticonvulsants SABRIL (vigabatrin) None Depressant XYREM (sodium oxybate) 3 bottles (540 mL)/30 days Neurotoxins BOTOX (onabotulinumtoxinA) None DYSPORT (abobotulinumtoxinA) None MYOBLOC (rimabotulinumtoxinB) None XEOMIN (incobotulinumtoxinA) None Parkinson's APOKYN (apomorphine) None Sleep Disorder HETLIOZ (tasimelteon) 1 cap/day Dermatology Alkylating Agents VALCHLOR (mechlorethamine) Gel None Endocrinology & Metabolism Gonadotropins ELIGARD (leuprolide) 22.5 mg (3-month) 1 -

Drugs Contraindicated in Pregnancy
DRUGS CONTRAINDICATED IN PREGNANCY (Part 1 of 2) This chart represents information on select drugs that are contraindicated (Pregnancy category X) for women who are pregnant. This is not an inclusive list of products that carry that pregnancy category. Those drugs that are contraindicated at a certain phase of the pregnancy are listed next to the product name. For more information on specific drug monographs, see product entries or consult the manufacturer. ALLERGIC DISORDERS Ortho Tri-Cyclen 28 (norgestimate/ INFECTIONS & INFESTATIONS Vistaril (hydroxyzine) Early pregnancy ethinyl estradiol) Bactrim (sulfamethoxazole/trimethoprim) Propecia (finasteride) 3rd trimester CARDIOVASCULAR SYSTEM Silvadene (silver sulfadiazine) Copegus (ribavirin) Aggrenox (dipyridamole/aspirin) Late pregnancy Flagyl (metronidazole) 1st trimester for 3rd trimester Solaraze (diclofenac sodium) trichomoniasis Altoprev (lovastatin) 3rd trimester Furadantin (nitrofurantoin) Pregnancy Bayer (aspirin) 3rd trimester Soriatane (acitretin) at term Caduet (amlodipine/atorvastatin) Sotret (isotretinoin) Grifulvin V (griseofulvin) Coumadin (warfarin sodium) SSD (silver sulfadiazine) Gris-Peg (griseofulvin) Crestor (rosuvastatin) Late pregnancy Macrobid (nitrofurantoin as Ecotrin (aspirin) 3rd trimester SSD AF (silver sulfadiazine) macrocrystals and monohydrate) Pregnancy at term Lescol (fluvastatin) Late pregnancy Macrodantin (nitrofurantoin Lescol XL (fluvastatin) Tazorac (tazarotene) macrocrystals) Pregnancy at term Letairis (ambrisentan) Tilia Fe (norethindrone acetate/ethinyl -

2019 Clinically Preferred Drug List 4Th Quarter Edition
2019 Clinically Preferred Drug List 4th Quarter Edition Last Revised: 10/15/2019 Version 2019Q4b Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) AGENTS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Agents 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) AGENTS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Agents 17 Fibrates 17 BIOSIMILARS 7 Other Agents 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC AGENTS 18 ANTIBIOTICS 8 KIDNEY & URINARY / UROLOGICAL AGENTS 18 Penicillins & Cephalosporins 8 Benign Prostate Hyperplasia 18 Tetracyclines 8 Urologic Agents / Others 18 Macrolides & Clindamycins 8 Erectile Dysfunction Agents 18 Sulfonamides, Sulfones & Ketolides 8 Gout Agents – Purine Inhibitors 19 Quinolones 8 Urinary Ph Modifiers 19 Miscellaneous Antibiotics 8 Potassium & Electrolytes 19 Phosphorus/Calcium/Electrolyte Depleters 19 ANTI-VIRALS 9 General Antivirals 9 OSTEOPOROSIS (BONE) AGENTS 20 HIV Antiviral Agents 9 HIV Pre-Exposure Propylaxis Agents 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) AGENTS 20 Anti-Inflammatory Agents (NSAIDS) 20 ANTI-INFECTIVES 10 COX-II Agents 21 Anaerobic Anti-Infectives 10 Analgesics, Narcotics (Opioids) 21 Antiparasitics 10 Analgesics, Salicylates & Non-Salicylates 21 Antimalarials & Antiprotozoals 10 Antihelmintic Agents 10 CENTRAL NERVOUS SYSTEM AGENTS 22 Anti-Anxiety Agents (Benzodiazepines) 22 ANTIEMETICS 10 Sedative/Sleeping Agents 22 A.D.D.