Prior Authorization — Select
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updated: May 20, 2021 Prior Authorization Drugs (NEW ADDITIONS HIGHLIGHTED)
Updated: May 20, 2021 Prior Authorization Drugs (NEW ADDITIONS HIGHLIGHTED) A Abatacept (ORENCIA) Abemaciclib (VERZENIO) Abiraterone Acetate (ZYTIGA) Abiraterone Acetate (+ GENERIC FORMULATIONS) AbobotulinumtoxinA (DYSPORT) Abraxane (ABRAXANE) Acalabrutinib (CALQUENCE) Actemra (ACTEMRA) Adalimumab (AMGEVITA) Adalimumab (HADLIMA) Adalimumab (HULIO) Adalimumab (HUMIRA) Adalimumab (HYRIMOZ) Adalimumab (IDACIO) Adcetris (ADCETRIS) Adcirca (ADCIRCA) Adempas (ADEMPAS) Afatinib (GIOTRIF) Afinitor (AFINITOR) Aflibercept (EYLEA) Aflibercept (ZALTRAP) Afstyla (AFSTYLA) Agalsidase Alfa (REPLAGAL) Agalsidase Beta (FABRAZYM) Aimovig (AIMOVIG) Ajovy (FREMANEZUMAB) Aldesleukin (PROLEUKIN) Aldurazyme (ALDURAZYME) Alecensaro (ALECENSARO) Alectinib (ALECENSARO) Alefacept (AMEVIVE) Alemtuzumab (MABCAMPATH) Alemtuzumab (LEMTRADA) Alglucosidase Alfa (MYOZYME) Alimta (ALIMTA) Alirocumab (PRALUENT) Alpha-1-Proteinase Inhibitor (PROLASTIN - C) Alpha-1-Proteinase Inhibitor (ZEMAIRA) Alpelisib (PIQRAY) Alunbrig (ALUNBRIG) Ambrisentan (VOLIBRIS) Ambrisentan (Generic Formulations) Amevive (AMEVIVE) Amgevita (ADALIMUMAB) Amifampridine Phosphate (FIRDAPSE) Amifampridine Phosphate (RUZURGI) Anakinra (KINERET) Apalutamide (ERLEADA) Apomorphine (KYNMOBI) Apremilast (OTEZLA) Page 1 of 17 Updated: May 20, 2021 Arsenic Trioxide (TRISENOX) Arsenic Trioxide (Generic Formulations) Arzerra (ARZERRA) Asfotase Alfa (STRENSIQ) Asparaginase (ERWINASE) Asunaprevir (SUNVEPRA) Atezolizumab (TECENTRIQ) Atriance (ATRIANCE) Aubagio (AUBAGIO) Avastin (AVASTIN) Avelumab (BAVENCIO) Avonex -

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Acne Class Update
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-2596 Drug Class Update with New Drug Evaluation: Acne Drugs Date of Review: June 2020 Date of Last Review: November 2018 Dates of Literature Search: 08/03/2018 - 12/26/2019 Generic Name: Trifarotene Brand Name (Manufacturer): Aklief® (Galderma Laboratories, LP) Dossier Received: no Current Status of PDL Class: See Appendix 1. Purpose for Class Update: The acne class has had one new approval, trifarotene cream, and several new product formulations since it was last reviewed in 2018. The purpose of this update is to evaluate new comparative evidence for trifarotene cream for the treatment of acne vulgaris and any new data on comparative efficacy or harms in the acne class since the previous update. Acne conglobata, acne fulminans, and severe cystic acne are covered conditions under the Oregon Health Plan (OHP). Research Questions: 1. What is the comparative efficacy and effectiveness of treatments for severe acne (topical agents of adapalene, adapalene/benzoyl peroxide, tretinoin, tazarotene, benzoyl peroxide, salicylic acid, dapsone, azelaic acid, clindamycin, erythromycin, minocycline, sulfacetamide, trifarotene; oral systemic antibiotics of doxycycline, minocycline, tetracycline, azithromycin, erythromycin, clindamycin, trimethoprim, and sulfamethoxazole/trimethoprim; hormonal agents of oral contraceptives and spironolactone; and oral isotretinoin)? 2. What are the comparative harms of treatments for severe acne? 3. Are there subpopulations of patients in which a particular treatment for severe acne would be more effective or associated with less harm? Conclusions: There are no new high-quality clinical practice guidelines which have evaluated comparative efficacy and safety of treatments for acne vulgaris. -

Duchenne Muscular Dystrophy (DMD) Agents
Therapeutic Class Overview Duchenne muscular dystrophy (DMD) Agents INTRODUCTION • Duchenne muscular dystrophy (DMD) is 1 of 4 conditions known as dystrophinopathies, which are inherited, X-linked myopathic disorders due to a defect in the dystrophin gene that results in the primary pathologic process of muscle fiber degradation. The hallmark symptom is progressive weakness (Darras 2018[a], Darras 2018[b], Muscular Dystrophy Association [MDA] 2019). The other 3 conditions include: Becker muscular dystrophy (BMD), which is a mild form of DMD; an intermediate presentation between BMD and DMD; and DMD-associated dilated cardiomyopathy, which has little or no clinical ○ skeletal or muscle disease (MDA 2019). • DMD symptom onset is in early childhood, usually between the ages of 2 and 3 years old. The proximal muscles are affected first, followed by the distal limb muscles. Generally, the lower external muscles will be affected before the upper. The affected child may have difficulties jumping, walking, and running (MDA 2019). • The prevalence of DMD ranges from 1 to 2 per 10,000 live male births; female-manifesting carriers are rarer, but can present with a range of symptoms that vary in their severities (Birnkrant et al 2018, Darras 2018[a], Emflaza Food and Drug Administration [FDA] Medical Review 2017). • The clinical course and lifespan of patients with DMD is relatively short. Individuals are usually confined to a wheelchair by age 13, and many die in their late teens or twenties from respiratory insufficiency or cardiomyopathy. Although survival until adulthood is more common now, very few patients survive past the 3rd decade (Darras 2018[a]). -

Vyondys 53™ (Golodirsen)
UnitedHealthcare® Commercial Medical Benefit Drug Policy Vyondys 53™ (Golodirsen) Policy Number: 2021D0088C Effective Date: April 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policy Coverage Rationale ....................................................................... 1 • Provider Administered Drugs – Site of Care Applicable Codes .......................................................................... 2 Background.................................................................................... 2 Community Plan Policy Benefit Considerations .................................................................. 3 • Vyondys 53™ (Golodirsen) Clinical Evidence ........................................................................... 3 U.S. Food and Drug Administration ............................................. 3 References ..................................................................................... 4 Policy History/Revision Information ............................................. 4 Instructions for Use ....................................................................... 4 Coverage Rationale See Benefit Considerations Vyondys 53 (golodirsen) may be covered for the treatment of Duchenne muscular dystrophy (DMD) in patients who meet all of the following criteria: For initial therapy, all of the following: o Diagnosis of Duchenne muscular dystrophy by, or in consultation with, a neurologist with expertise in the diagnosis of DMD; and o Submission of medical records (e.g., chart notes, laboratory -

761094Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761094Orig1s000 CLINICAL REVIEW(S) Medical Officer’s Review of BLA 761094 Review #2 BLA 761094 Submission Date: July 19, 2018 Receipt Date: July 19, 2018 Review Date: July 23, 2018 Applicant: Dompé farmaceutici S.p.A. Via Santa Lucia 6-20122 Milan, Italy Applicant’s Representative: Lamberto Dionigi. Regulatory Affairs & Drug Safety Director 39-02-5838-3559 Drug: OXERVATE (cenegermin – bkbj) ophthalmic solution, 20 mcg/mL Submitted: Reference is made to the meeting Teleconference of 15-July-2018, in which the FDA has requested to provide information available at Dompé on the use of Oxervate in the pediatric population. Pediatric patients exposed to Oxervate: Dompé confirms that Oxervate is not yet approved in Europe for use in children and no clinical studies have been conducted in this population. As far as the company is aware, four (4) Neurotrophic Keratitis (NK) pediatric patients, aged from 2 to 12 years of age, have been exposed to Oxervate. A 6-year-old child with severe NK secondary to Stuve-Wiedmann syndrome was treated with Oxervate under “Temporary Authorization for Use” (ATU) in France. Dompé reports that the corneal ulcer was completely healed at the end of the treatment. There were signs of corneal sensitivity recovery by month 8 post-treatment. A 9-year-old child with NK was treated with Oxervate under ATU in France. Dompé reports that the lesion improved, but did not completely heal with the treatment. There is no other follow-up information available. A 12-year-old child with severe NK was treated with Oxervate under Expanded Access in the US. -

Xifaxan® (Rifaximin)
Wednesday, October 14, 2015 4 p.m. Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review Board Members FROM: Bethany Holderread, Pharm.D. SUBJECT: Packet Contents for Board Meeting – October 14, 2015 DATE: October 1, 2015 NOTE: The DUR Board will meet at 4:00 p.m. The meeting will be held at 4345 N Lincoln Blvd. Enclosed are the following items related to the October meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Action Item – Vote on 2016 Meeting Dates – Appendix B Update on Medication Coverage Authorization Unit/Bowel Preparation Medication Post-Educational Mailing – Appendix C Action Item – Vote to Prior Authorize Tykerb® (Lapatinib), Halaven® (Eribulin), Ixempra® (Ixabepilone), Kadcyla® (Ado-Trastuzumab), Afinitor® (Everolimus), & Perjeta® (Pertuzumab) – Appendix D Action Item – Vote to Prior Authorize Orkambi™ (Lumacaftor/Ivacaftor) – Appendix E Action Item – Vote to Prior Authorize Savaysa® (Edoxaban) – Appendix F Action Item – Vote to Prior Authorize Epanova® (Omega-3-Carboxylic Acids), Praluent® (Alirocumab), & Repatha™ (Evolocumab) – Appendix G Annual Review of Constipation and Diarrhea Medications and 30-Day Notice to Prior Authorize Movantik™ (Naloxegol), Viberzi™ (Eluxadoline), & Xifaxan® (Rifaximin) – Appendix H 30-Day Notice to Prior Authorize Daraprim® (Pyrimethamine) – Appendix I Annual Review of Allergy Immunotherapies and 30-Day Notice to Prior Authorize Oralair® (Sweet Vernal, Orchard, Perennial Rye, Timothy, & Kentucky Blue Grass Mixed Pollens Allergen Extract) – Appendix J Annual Review of Non-Steroidal Anti-Inflammatory Drugs and 30-Day Notice to Prior Authorize Dyloject™ (Diclofenac Sodium) – Appendix K ORI-4403 • P.O. -

Saizen Prior Authorization Request Form (Page 1 of 4) DO NOT COPY for FUTURE USE
OptumRx has partnered with CoverMyMeds to receive prior authorization requests, saving you time and often delivering real-time determinations. Visit go.covermymeds.com/OptumRx to begin using this free service. Please note: All information below is required to process this request. Mon-Fri: 5am to 10pm Pacific / Sat: 6am to 3pm Pacific ® Saizen Prior Authorization Request Form (Page 1 of 4) DO NOT COPY FOR FUTURE USE. FORMS ARE UPDATED FREQUENTLY AND MAY BE BARCODED Member Information (required) Provider Information (required) Member Name: Provider Name: Insurance ID#: NPI#: Specialty: Date of Birth: Office Phone: Street Address: Office Fax: City: State: Zip: Office Street Address: Phone: City: State: Zip: Medication Information (required) Medication Name: Strength: Dosage Form: Check if requesting brand Directions for Use: Check if request is for continuation of therapy Clinical Information (required) Select the diagnosis below: Pediatric growth hormone deficiency Growth hormone deficiency in adults Growth hormone deficiency in transition phase adolescents Isolated growth hormone deficiency in adults Pediatric growth failure associated with chronic renal insufficiency Prader-Willi syndrome Short-stature homeobox (SHOX) gene deficiency Small for gestational age (SGA) Turner syndrome or Noonan syndrome Other diagnosis: _________________________________________ ICD-10 Code(s): ___________________________________ Clinical Information: Select if the requested medication is prescribed by or in consultation with one of the following -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -
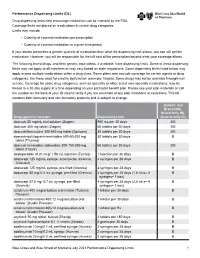
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral