2021 National Formulary 3Rd Quarter Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anthem Blue Cross Drug Formulary
Erythromycin/Sulfisoxazole (generic) INTRODUCTION Penicillins ...................................................................... Anthem Blue Cross uses a formulary Amoxicillin (generic) (preferred list of drugs) to help your doctor Amoxicillin/Clavulanate (generic/Augmentin make prescribing decisions. This list of drugs chew/XR) is updated quarterly, by a committee Ampicillin (generic) consisting of doctors and pharmacists, so that Dicloxacillin (generic) the list includes drugs that are safe and Penicillin (generic) effective in the treatment of diseases. If you Quinolones ..................................................................... have any questions about the accessibility of Ciprofloxacin/XR (generic) your medication, please call the phone number Levofloxacin (Levaquin) listed on the back of your Anthem Blue Cross Sulfonamides ................................................................ member identification card. Erythromycin/Sulfisoxazole (generic) In most cases, if your physician has Sulfamethoxazole/Trimethoprim (generic) determined that it is medically necessary for Sulfisoxazole (generic) you to receive a brand name drug or a drug Tetracyclines .................................................................. that is not on our list, your physician may Doxycycline hyclate (generic) indicate “Dispense as Written” or “Do Not Minocycline (generic) Substitute” on your prescription to ensure Tetracycline (generic) access to the medication through our network ANTIFUNGAL AGENTS (ORAL) _________________ of community -
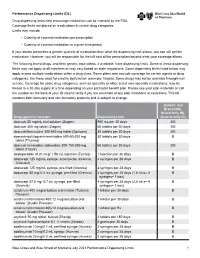
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -
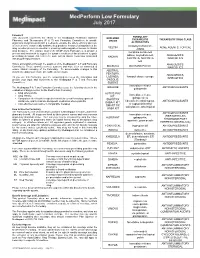
Medperform Low Formulary July 2017
MedPerform Low Formulary July 2017 Foreword FORMULARY This document represents the efforts of the MedImpact Healthcare Systems EXCLUDED THERAPEUTIC THERAPEUTIC DRUG CLASS Pharmacy and Therapeutics (P & T) and Formulary Committees to provide DRUGS physicians and pharmacists with a method to evaluate the safety, efficacy and cost- ALTERNATIVES effectiveness of commercially available drug products. A structured approach to the clindamycin/tretinoin, VELTIN ACNE AGENTS, TOPICAL drug selection process is essential in ensuring continuing patient access to rational ZIANA drug therapies. The ultimate goal of the MedPerform Formulary is to provide a morphine sulfate ER process and framework to support the dynamic evolution of this document to guide tablets, oxycodone ER, ANALGESICS, prescribing decisions that reflect the most current clinical consensus associated KADIAN with drug therapy decisions. NUCYNTA, NUCYNTA NARCOTICS ER This is accomplished through the auspices of the MedImpact P & T and Formulary ANALGESICS, BELBUCA BUTRANS PATCH Committees. These committees meet quarterly and more often as warranted to NARCOTICS ensure clinical relevancy of the Formulary. To accommodate changes to this ABSTRAL, document, updates are made accessible as necessary. FENTORA, ANALGESICS, LAZANDA, fentanyl citrate lozenge As you use this Formulary, you are encouraged to review the information and NARCOTICS provide your input and comments to the MedImpact P & T and Formulary ONSOLIS, Committees. SUBSYS immediate-release GRALISE ANTICONVULSANTS The MedImpact -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees. -

Estrogens, Estrogen Agonists/Antagonists, and Calcitonin Nelson B
48 Estrogens, Estrogen Agonists/Antagonists, and Calcitonin Nelson B. Watts Estrogen 408 Calcitonin 409 Estrogen Agonists/Antagonists (Selective Estrogen Receptor Summary 410 Modulators or Serms) 409 Selected Readings 410 Thus, it appears that estrogen has protective effects on ESTROGEN the skeleton only for as long as it is taken. However, women who take estrogen and then stop should be no It is well established that osteoporosis is more common worse off compared with women who have never taken in women than men and fracture risk increases dramati- estrogen at all. cally after menopause. The relationship between estro- Following results of the WHI, estrogen treatment is gen defi ciency and osteoporosis was fi rst suggested in the recommended for relief of menopausal symptoms, but in mid-1900s when Albright showed that treatment with the lowest dose and for the shortest time necessary. Most estrogen reversed the negative calcium balance in post- women are not suffi ciently symptomatic at menopause menopausal women. The positive effects of estrogen on to require estrogen therapy, and most who require estro- peak bone mass and on bone loss have been demon- gen will be able to stop after a few years. However, a strated in both men and women. For years, estrogen was minority will require estrogen long-term. For those considered the treatment of choice for postmenopausal women, estrogen may be suffi cient for prevention and/or women and widely advocated for prevention of bone loss, treatment of osteoporosis. with numerous trials showing prevention of bone loss Several different forms of estrogen are available for in recently menopausal women and increased of bone therapeutic use (e.g., estradiol, conjugated estrogen ester- mineral density (BMD) in women with postmenopausal ifi ed estrogens, etc.) as well as several routes of adminis- osteoporosis. -

Specialty Guideline Management
SPECIALTY GUIDELINE MANAGEMENT North Carolina State Health Plan: Fertility Agents PROGRAM RATIONALE Client Requested: The intent of the criteria is to ensure that patients follow selection elements established by North Carolina State Health Plan’s Commercial Prior Authorization Approval policy. PRIOR AUTHORIZATION CRITERIA1 • Coverage is provided for female infertility treatment and in males for non-infertility indications. • Coverage is NOT provided for patients using fertility medication in conjunction with any type of Artificial Reproductive Technology (ART) procedure. ART procedures include In Vitro Fertilization (IVF), Gamete Intrafallopian Transfer (GIFT), Zygote Intrafallopian Transfer (ZIFT) and Intrauterine or Artificial Insemination. COVERED FERTILITY AGENTS* Medication Generic Name Covered Indications Gonadotropins Follicle Stimulating Hormone (FSH) Bravelle† urofollitropin . Ovulation induction Follistim AQ follitropin beta . Ovulation induction . Hypogonadotropic hypogonadism in males Gonal-F†/ follitropin alfa . Ovulation induction Gonal-F RFF Pen† . Hypogonadotropic hypogonadism in males (Gonal-F only) Human Chorionic Gonadotropin (hCG) Novarel, Pregnyl, chorionic gonadotropin . Ovulation induction hcG (generic) . Selected cases of hypogonadotropic hypogonadism in males (ie, hypogonadism secondary to a pituitary deficiency) . Prepubertal cryptorchidism Ovidrel choriogonadotropin alfa . Ovulation induction Human Menopausal Gonadotropin (hMG) Menopur menotropin . Ovulation induction Gonadotropin Releasing Hormone (GnRH) Analogs -

ART Drugs Page: 1 of 8
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.02 Section: Prescription Drugs Effective Date: July 1, 2021 Subsection: Endocrine and Metabolic Drugs Original Policy Date: January 1, 2011 Subject: ART Drugs Page: 1 of 8 Last Review Date: June 17, 2021 ART Drugs Description Bravelle (urofollitropin) Cetrotide (cetrorelix) Clomid, Clomiphene Powder, Serophene (clomiphene citrate) Crinone, Endometrin, Progesterone in Oil, Progesterone Powder, Prometrium (progesterone) Follistim AQ (follitropin beta) Gonal-F, Gonal F RFF (follitropin alfa) Ganirelix (ganirelix) Menopur (menotropins) Milprosa (progesterone) Background Assisted Reproductive Technologies (ART) represent a group of non-coital manipulations and processes that manipulate ova and/or sperm to achieve a pregnancy. The most well-known examples are ovulation induction, intrauterine insemination and in-vitro fertilization. ART and infertility drugs used in conjunction with ART procedures or erectile or sexual dysfunction, weight loss, performance enhancement and anti-aging are not covered benefits. The diagnosis of hypogonadotropic hypogonadism is an off-label indication for these medications. A variety of drugs are used to manipulate the hypothalamic-pituitary-gonadal axis in order to induce ovulation in females known as controlled ovarian hyperstimulation (COH). Some of these pharmacologic agents are used for additional clinical care indications. Drugs Included in Infertility Drugs / ART Criteria • Antagon (ganirelix) – inhibition -

AHFS Therapeutic
AHFS Therapeutic AHFS Therapeutic Class Description HICL HICL Description Program Edit Criteria 1 Edit Criteria 2 Edit Criteria 3 Edit Criteria 4 Edit Criteria 5 Class 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 0.667000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 0.750000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 1.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 2.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 2.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 3.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 3.750000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 6.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 7.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE Medical Exception Required 282032 RESPIRATORY AND CNS STIMULANTS 000007 CAFFEINE/DEXTROSE PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000008 CAFFEINE/MULTIVITAMIN PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000009 CAFFEINE/ETHYL ALCOHOL PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000010 CAFFEINE/SODIUM BENZOATE PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000011 CAFFEINE CITRATE -

Appendiks Til Marte Myhre Reigstad, Inger Kristin Larsen, Ritsa Storeng
Appendiks til Marte Myhre Reigstad, Inger Kristin Larsen, Ritsa Storeng. Kreftrisiko hos mor og barn etter fertilitetsbehandling. Tidsskr Nor Legeforen 2018; 138. doi: 10.4045/tidsskr.17.1098. Dette appendikset er et tillegg til artikkelen og er ikke bearbeidet redaksjonelt. Ramme 1 Beskrivelse av søkestrengen Søket består av 3 deler; fertilitetsbehandling, risiko, kreft som er kombinert med AND. Det er søkt med kontrollerte emneord (MeSH eller EMTREE), ord i tittel/abstakt /forfatters nøkkelord, kombinert med OR, innenfor de tre delene. EMBASE: artificial insemination/ or embryo disposition/ or exp embryo transfer/ or fertilization in vitro/ or in vitro oocyte maturation/ or intracytoplasmic sperm injection/ or ovulation induction/ or in vitro oocyte maturation/ or intracytoplasmic sperm injection/ or ovulation induction/ or superovulation/ or chorionic gonadotropin/ or clomifene/ or clomifene citrate/ or corifollitropin alfa/ or follitropin/ or gonadotropin/ or human menopausal gonadotropin/ or recombinant chorionic gonadotropin/ or recombinant follitropin/ or recombinant follitropin plus recombinant luteinizing hormone/ or recombinant luteinizing hormone/ or urofollitropin/ or gonadorelin/ or (gonadotropin* or clomifene or clomiphene or corifollitropin* or follitropin* or recombinant luteinizing hormone or urofollitropin* or gonadorelin* or buserelin* or leuprolide or menotropin* or nafarelin* or (fertilization* adj3 vitro) or (fertilisation* adj3 vitro) or (fertilization* adj3 invitro) or (fertilisation* adj3 invitro) or ivf or intracytoplasmic