REVIEW Estrogens and Atherosclerosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anthem Blue Cross Drug Formulary
Erythromycin/Sulfisoxazole (generic) INTRODUCTION Penicillins ...................................................................... Anthem Blue Cross uses a formulary Amoxicillin (generic) (preferred list of drugs) to help your doctor Amoxicillin/Clavulanate (generic/Augmentin make prescribing decisions. This list of drugs chew/XR) is updated quarterly, by a committee Ampicillin (generic) consisting of doctors and pharmacists, so that Dicloxacillin (generic) the list includes drugs that are safe and Penicillin (generic) effective in the treatment of diseases. If you Quinolones ..................................................................... have any questions about the accessibility of Ciprofloxacin/XR (generic) your medication, please call the phone number Levofloxacin (Levaquin) listed on the back of your Anthem Blue Cross Sulfonamides ................................................................ member identification card. Erythromycin/Sulfisoxazole (generic) In most cases, if your physician has Sulfamethoxazole/Trimethoprim (generic) determined that it is medically necessary for Sulfisoxazole (generic) you to receive a brand name drug or a drug Tetracyclines .................................................................. that is not on our list, your physician may Doxycycline hyclate (generic) indicate “Dispense as Written” or “Do Not Minocycline (generic) Substitute” on your prescription to ensure Tetracycline (generic) access to the medication through our network ANTIFUNGAL AGENTS (ORAL) _________________ of community -

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -
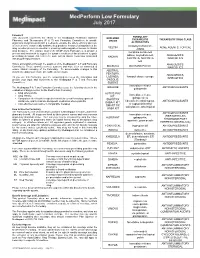
Medperform Low Formulary July 2017
MedPerform Low Formulary July 2017 Foreword FORMULARY This document represents the efforts of the MedImpact Healthcare Systems EXCLUDED THERAPEUTIC THERAPEUTIC DRUG CLASS Pharmacy and Therapeutics (P & T) and Formulary Committees to provide DRUGS physicians and pharmacists with a method to evaluate the safety, efficacy and cost- ALTERNATIVES effectiveness of commercially available drug products. A structured approach to the clindamycin/tretinoin, VELTIN ACNE AGENTS, TOPICAL drug selection process is essential in ensuring continuing patient access to rational ZIANA drug therapies. The ultimate goal of the MedPerform Formulary is to provide a morphine sulfate ER process and framework to support the dynamic evolution of this document to guide tablets, oxycodone ER, ANALGESICS, prescribing decisions that reflect the most current clinical consensus associated KADIAN with drug therapy decisions. NUCYNTA, NUCYNTA NARCOTICS ER This is accomplished through the auspices of the MedImpact P & T and Formulary ANALGESICS, BELBUCA BUTRANS PATCH Committees. These committees meet quarterly and more often as warranted to NARCOTICS ensure clinical relevancy of the Formulary. To accommodate changes to this ABSTRAL, document, updates are made accessible as necessary. FENTORA, ANALGESICS, LAZANDA, fentanyl citrate lozenge As you use this Formulary, you are encouraged to review the information and NARCOTICS provide your input and comments to the MedImpact P & T and Formulary ONSOLIS, Committees. SUBSYS immediate-release GRALISE ANTICONVULSANTS The MedImpact -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees. -

Estrogens, Estrogen Agonists/Antagonists, and Calcitonin Nelson B
48 Estrogens, Estrogen Agonists/Antagonists, and Calcitonin Nelson B. Watts Estrogen 408 Calcitonin 409 Estrogen Agonists/Antagonists (Selective Estrogen Receptor Summary 410 Modulators or Serms) 409 Selected Readings 410 Thus, it appears that estrogen has protective effects on ESTROGEN the skeleton only for as long as it is taken. However, women who take estrogen and then stop should be no It is well established that osteoporosis is more common worse off compared with women who have never taken in women than men and fracture risk increases dramati- estrogen at all. cally after menopause. The relationship between estro- Following results of the WHI, estrogen treatment is gen defi ciency and osteoporosis was fi rst suggested in the recommended for relief of menopausal symptoms, but in mid-1900s when Albright showed that treatment with the lowest dose and for the shortest time necessary. Most estrogen reversed the negative calcium balance in post- women are not suffi ciently symptomatic at menopause menopausal women. The positive effects of estrogen on to require estrogen therapy, and most who require estro- peak bone mass and on bone loss have been demon- gen will be able to stop after a few years. However, a strated in both men and women. For years, estrogen was minority will require estrogen long-term. For those considered the treatment of choice for postmenopausal women, estrogen may be suffi cient for prevention and/or women and widely advocated for prevention of bone loss, treatment of osteoporosis. with numerous trials showing prevention of bone loss Several different forms of estrogen are available for in recently menopausal women and increased of bone therapeutic use (e.g., estradiol, conjugated estrogen ester- mineral density (BMD) in women with postmenopausal ifi ed estrogens, etc.) as well as several routes of adminis- osteoporosis. -

Genome-Wide Identification of Brain Mirnas in Response to High
Zhao et al. BMC Molecular Biol (2019) 20:3 https://doi.org/10.1186/s12867-019-0120-4 BMC Molecular Biology RESEARCH ARTICLE Open Access Genome‑wide identifcation of brain miRNAs in response to high‑intensity intermittent swimming training in Rattus norvegicus by deep sequencing Yanhong Zhao1*†, Anmin Zhang2,3*†, Yanfang Wang4, Shuping Hu3, Ruiping Zhang2 and Shuaiwei Qian2 Abstract Background: Physical exercise can improve brain function by altering brain gene expression. The expression mecha- nisms underlying the brain’s response to exercise still remain unknown. miRNAs as vital regulators of gene expres- sion may be involved in regulation of brain genes in response to exercise. However, as yet, very little is known about exercise-responsive miRNAs in brain. Results: We constructed two comparative small RNA libraries of rat brain from a high-intensity intermittent swim- ming training (HIST) group and a normal control (NC) group. Using deep sequencing and bioinformatics analysis, we identifed 2109 (1700 from HIST, 1691 from NC) known and 55 (50 from HIST, 28 from NC) novel candidate miRNAs. Among them, 34 miRNAs were identifed as signifcantly diferentially expressed in response to HIST, 16 were up- regulated and 18 were down-regulated. The results showed that all members of mir-200 family were strongly up- regulated, implying mir-200 family may play very important roles in HIST response mechanisms of rat brain. A total of 955 potential target genes of these 34 exercise-responsive miRNAs were identifed from rat genes. Most of them are directly involved in the development and regulatory function of brain or nerve. -

Growth Cone Responses to Growth and Chemotropic Factors
European Journal of Neuroscience European Journal of Neuroscience, Vol. 28, pp. 268–278, 2008 doi:10.1111/j.1460-9568.2008.06327.x Growth cone responses to growth and chemotropic factors Staci D. Sanford,1 Jesse C. Gatlin,1,* Tomas Ho¨kfelt2 and Karl H. Pfenninger1 1Departments of Pediatrics, and of Cell and Developmental Biology, and Neuroscience Program, University of Colorado School of Medicine, and University of Colorado Cancer Center, Aurora, CO 80045, USA 2Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden Keywords: attractants, axonal pathfinding, growth cone, growth promotion, neuropeptides, repellents Abstract During nervous system development axons reach their target areas under the influence of numerous guidance cues that affect rate and direction of growth. This report addresses the unsettled question of whether and to what extent growth velocity and turning responses (attraction, repulsion) are interdependent. We exposed individual growth cones of fetal rat dorsal root ganglion neurons in culture asymmetrically to gradients of seven different factors and recorded their growth rates and turning angles. Growth cones exhibited divergent patterns of turning and growth responses. For example, hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1) and thrombin all promoted growth, but HGF was a powerful attractant, thrombin a potent repellent and IGF-1 did not elicit turning. Galanin and neuropeptide Y also affected growth and ⁄ or turning differentially. Finally, nerve growth factor in the culture -

Permeability Due to Drug Binding Kinetics and Reactivation Among
Differential Risk of Tuberculosis Reactivation among Anti-TNF Therapies Is Due to Drug Binding Kinetics and Permeability This information is current as of September 26, 2021. Mohammad Fallahi-Sichani, JoAnne L. Flynn, Jennifer J. Linderman and Denise E. Kirschner J Immunol 2012; 188:3169-3178; Prepublished online 29 February 2012; doi: 10.4049/jimmunol.1103298 Downloaded from http://www.jimmunol.org/content/188/7/3169 Supplementary http://www.jimmunol.org/content/suppl/2012/02/29/jimmunol.110329 http://www.jimmunol.org/ Material 8.DC1 References This article cites 59 articles, 21 of which you can access for free at: http://www.jimmunol.org/content/188/7/3169.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2012 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Differential Risk of Tuberculosis Reactivation among Anti-TNF Therapies Is Due to Drug Binding Kinetics and Permeability Mohammad Fallahi-Sichani,*,1 JoAnne L. -

AHFS Therapeutic
AHFS Therapeutic AHFS Therapeutic Class Description HICL HICL Description Program Edit Criteria 1 Edit Criteria 2 Edit Criteria 3 Edit Criteria 4 Edit Criteria 5 Class 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 0.667000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 0.750000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 1.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 2.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 2.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 3.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 3.750000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 6.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 7.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE Medical Exception Required 282032 RESPIRATORY AND CNS STIMULANTS 000007 CAFFEINE/DEXTROSE PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000008 CAFFEINE/MULTIVITAMIN PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000009 CAFFEINE/ETHYL ALCOHOL PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000010 CAFFEINE/SODIUM BENZOATE PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000011 CAFFEINE CITRATE -

Network-Based Characterization of Drug-Protein Interaction Signatures
Tabei et al. BMC Systems Biology 2019, 13(Suppl 2):39 https://doi.org/10.1186/s12918-019-0691-1 RESEARCH Open Access Network-based characterization of drug-protein interaction signatures with a space-efficient approach Yasuo Tabei1*, Masaaki Kotera2, Ryusuke Sawada3 and Yoshihiro Yamanishi3,4 From The 17th Asia Pacific Bioinformatics Conference (APBC 2019) Wuhan, China. 14–16 January 2019 Abstract Background: Characterization of drug-protein interaction networks with biological features has recently become challenging in recent pharmaceutical science toward a better understanding of polypharmacology. Results: We present a novel method for systematic analyses of the underlying features characteristic of drug-protein interaction networks, which we call “drug-protein interaction signatures” from the integration of large-scale heterogeneous data of drugs and proteins. We develop a new efficient algorithm for extracting informative drug- protein interaction signatures from the integration of large-scale heterogeneous data of drugs and proteins, which is made possible by space-efficient representations for fingerprints of drug-protein pairs and sparsity-induced classifiers. Conclusions: Our method infers a set of drug-protein interaction signatures consisting of the associations between drug chemical substructures, adverse drug reactions, protein domains, biological pathways, and pathway modules. We argue the these signatures are biologically meaningful and useful for predicting unknown drug-protein interactions and are expected to contribute to rational drug design. Keywords: Drug-protein interaction prediction, Drug discovery, Large-scale prediction Background similar drugs are expected to interact with similar pro- Target proteins of drug molecules are classified into a pri- teins, with which the similarity of drugs and proteins are mary target and off-targets. -

L.A. CARE FORMULARY Healthy Families
L.A. CARE FORMULARY Healthy Families 2012 INTRODUCTION Foreword This document represents the efforts of L.A. Care Health Plan’s Pharmacy Therapeutics and New Technology Committee (PT&T) to provide physicians and pharmacists with a method to begin to evaluate the various drug products available. The medical treatment of patients is frequently relative to the practical application of drug therapy. Due to the vast availability of medication therapy and treatment modalities, a reasonable program of drug product selection and drug usage must be developed. The goal of the L.A. Care Formulary is to enhance the physician and pharmacist’s abilities to provide optimal cost effective drug therapy for patients. The development, maintenance, and improvement of this process are evolutionary and require constant attention. This is accomplished by the L.A. Care PT&T Committee. The Formulary is a continually reviewed and revised list of drugs, which mirror the prevailing clinical opinion of the PT&T Committee. Unfortunately, this dynamic process does not allow this document to be completely accurate at all times. To accommodate the necessary changes of this document, updates are available online at: http://www.lacare.org. As you use this Formulary, you are encouraged to review the information and provide your input and comments to the L.A. Care PT&T Committee. The L.A. Care PT&T Committee uses the following criteria in the evaluation of drug selection for the L.A. Care Formulary: • Drug safety profile • Drug efficacy • Drug effectiveness • Comparison of relevant drug benefits to current formulary agents of similar use, while minimizing duplications • Equitable cost and outcomes of the total cost of drug and medical care How to Use the Formulary The Formulary is a list of covered and preferred drug agents for L.A. -

Guidelines for Pharmacists Performing Clinical Interventions
Guidelines for pharmacists performing clinical interventions PSA Committed to better health © Pharmaceutical Society of Australia Ltd. 2018 This publication contains material that has been provided by the Pharmaceutical Society of Australia (PSA), and may contain material provided by the Commonwealth and third parties. Copyright in material provided by the Commonwealth or third parties belong to them. PSA owns the copyright in the publication as a whole and all material in the publication that has been developed by PSA. In relation to PSA owned material, no part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968 (Cwth), or the written permission of PSA. Requests and inquiries regarding permission to use PSA material should be addressed to: Pharmaceutical Society of Australia, PO Box 42, Deakin West ACT 2600. If you would like to use material that has been provided by the Commonwealth or third parties, contact them directly. Disclaimer Neither the PSA, nor any person associated with the preparation of this document, accepts liability for any loss which a user of this document may suffer as a result of reliance on the document and, in particular, for: • use of the Guidelines for a purpose for which they were not intended • any errors or omissions in the Guidelines • any inaccuracy in the information or data on which the Guidelines are based or which is contained in them • any interpretations or opinions stated in, or which may be inferred from, the Guidelines. Notification of any inaccuracy or ambiguity found in this document should be made without delay in order that the issue may be investigated and appropriate action taken.