Pharmacy Prior Authorization Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of Psychophysiological Motoric Reactivity Is Influenced By
Development of psychophysiological motoric reactivity is influenced by peripubertal pharmacological inhibition of gonadotropin releasing hormone action - Results of an ovine model Neil P. Evans, Jane Robinson J.E., Hans Erhard, Erik Ropstad, Lynne M. Fleming, Ira Ronit Hebold Haraldsen To cite this version: Neil P. Evans, Jane Robinson J.E., Hans Erhard, Erik Ropstad, Lynne M. Fleming, et al.. Develop- ment of psychophysiological motoric reactivity is influenced by peripubertal pharmacological inhibition of gonadotropin releasing hormone action - Results of an ovine model. Psychoneuroendocrinology, El- sevier, 2012, 37 (11), pp.1876-1884. 10.1016/j.psyneuen.2012.03.020. hal-01186791 HAL Id: hal-01186791 https://hal.archives-ouvertes.fr/hal-01186791 Submitted on 29 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. + Models PNEC-2163; No. of Pages 9 Psychoneuroendocrinology (2012) xxx, xxx—xxx Available online at www.sciencedirect.com j ournal homepage: www.elsevier.com/locate/psyneuen Development of psychophysiological motoric reactivity is influenced by peripubertal pharmacological inhibition of gonadotropin releasing § hormone action — Results of an ovine model a, a b c Neil P. Evans *, Jane E. Robinson , Hans W. Erhard , Erik Ropstad , a d Lynne M. -

III IIII USO05721278A United States Patent (19) 11) Patent Number: 5,721,278 Garfield Et Al
III IIII USO05721278A United States Patent (19) 11) Patent Number: 5,721,278 Garfield et al. 45) Date of Patent: Feb. 24, 1998 54 OWULATON CONTROL BY REGULATING 58) Field of Search ............................ 514/15, 121, 651, NTRC OXDE LEVELS 514/652,561, 841, 843, 648 Inventors: Robert E. Garfield, Friendswood; 56 References Cited Chandrasekhar Yallampalli, Houston, U.S. PATENT DOCUMENTS both of Tex. 4,338,305 7/1982 Corbin ................................... 424/177 73 Assignee: Board of Regents, The University of 4,851,385 7/1989 Rueske ..................................... 514/15 Texas System, Austin,Tex. 5,470,847 11/1995 Garfield et al. ......................... 514/171. Primary Examiner. Theodore J. Criares 21 Appl. No.: 477,187 Attorney, Agent, or Firm-Arnold, White & Durkee 22 Filed: Jun. 7, 1995 57 ABSTRACT Inhibition of ovulation in a female may be achieved by Related U.S. Application Data administering a nitric oxide synthase inhibitor, alone or in 62 Division of Ser. No. 165,309, Dec. 10, 1993, Pat. No. combination with one or more of a progestin, an estrogen, 5,470,847. and an LH-RH antagonist, thereby preventing conception. The stimulation of ovulation in a female may be achieved by 51) Int. Cl. ......... A61K 31/195; A61K 31/135: administering a nitric oxide source, optionally in further A61K 31/56 combination with one or more of clomiphene, a 52 U.S.C. ....................... 514/652; 514/171; 514/561; gonadotropin, and an LH-RH agonist. 514/563; 514/651; 514/565; 514/841; 514/843; 514/648 4 Claims, 1 Drawing Sheet U.S. Patent Feb. 24, 1998 5,721,278 HypothalamuS Anterior Pituitary Gland ProgesterOne Estrogen / \ CorpOra OVa lutea \(es j \s 63 O Graafian follicle Follicular Ovulatory Luteal PaSe Phase Phase FIG. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -
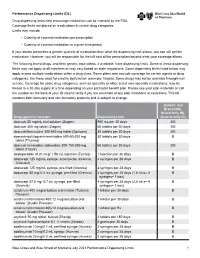
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Annual Report
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington D.C. 20549 FORM 10-K ANNUAL REPORT UNDER SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended: December 31, 2010 ! TRANSITION REPORT UNDER SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number: 333-150937 Curaxis Pharmaceutical Corporation (Exact name of registrant as specified in its charter) Nevada 26-1919261 (State or Other Jurisdiction of (I.R.S. Employer Identification No.) Incorporation or Organization) 51 Berkshire Street Swampscott, MA, 01907 (Address of principal executive offices) (919) 313-4930 (Registrant’s telephone number, including area code) Securities registered under Section 12(b) of the Exchange Act: None Securities registered under Section 12(g) of the Exchange Act: Common Stock, par value $0.0001 per share Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ! No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ! No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes No ! Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage -

2013 BCPS J.Pmd
REVIEW ARTICLES Endometriosis - An Update LP BANUa, S TASNIMb Summary: relevant information. The study results showed that both Endometriosis is a common and important health problem medical and surgical treatments are almost equally effective of women. The prevalence of endometriosis is difficult to in pain management there is no evidence that medical determine. Diagnosis is often difficult and delayed due to treatment improves fertility. Surgical treatment is associated close similarity of symptoms of endometriosis with other with small but significant improvement in live birth rate. gynecological disorder. Optimum treatment involves a Newer non hormonal drugs are more selective with less combination of medical and surgical treatments tailored to metabolic side effects. Surgical treatment is invasive the patient’s needs and response. procedure and repeated surgery is associated with major complication. The objective of the study is to discuss the current updates Medical treatment is considered more effective in the long on diagnosis, treatment and the optimal role of different term management of endometriosis. options in the treatment of endometriosis. The article reviews different medical journals, medline and internet to get the (J Banagladesh Coll Phys Surg 2013; 31: 144-149) Introduction: Endometriosis run in families. The mode of inheritance Endometriosis is a chronic and recurrent disease is probably polygenic and multi factorial. Chromosome characterized by the presence and proliferation of no 10 q26 5, 6 is thought to carry the involved gene. functional endometrial glands and stroma outside the There is some defect in immune response to tissue injury uterine cavity. Around 10% of women of reproductive in women with endometriosis that fail to remove age are affected by endometriosis, suffer from chronic refluxed menstrual debris from the extra uterine site. -

Specialty Guideline Management
SPECIALTY GUIDELINE MANAGEMENT North Carolina State Health Plan: Fertility Agents PROGRAM RATIONALE Client Requested: The intent of the criteria is to ensure that patients follow selection elements established by North Carolina State Health Plan’s Commercial Prior Authorization Approval policy. PRIOR AUTHORIZATION CRITERIA1 • Coverage is provided for female infertility treatment and in males for non-infertility indications. • Coverage is NOT provided for patients using fertility medication in conjunction with any type of Artificial Reproductive Technology (ART) procedure. ART procedures include In Vitro Fertilization (IVF), Gamete Intrafallopian Transfer (GIFT), Zygote Intrafallopian Transfer (ZIFT) and Intrauterine or Artificial Insemination. COVERED FERTILITY AGENTS* Medication Generic Name Covered Indications Gonadotropins Follicle Stimulating Hormone (FSH) Bravelle† urofollitropin . Ovulation induction Follistim AQ follitropin beta . Ovulation induction . Hypogonadotropic hypogonadism in males Gonal-F†/ follitropin alfa . Ovulation induction Gonal-F RFF Pen† . Hypogonadotropic hypogonadism in males (Gonal-F only) Human Chorionic Gonadotropin (hCG) Novarel, Pregnyl, chorionic gonadotropin . Ovulation induction hcG (generic) . Selected cases of hypogonadotropic hypogonadism in males (ie, hypogonadism secondary to a pituitary deficiency) . Prepubertal cryptorchidism Ovidrel choriogonadotropin alfa . Ovulation induction Human Menopausal Gonadotropin (hMG) Menopur menotropin . Ovulation induction Gonadotropin Releasing Hormone (GnRH) Analogs -

ART Drugs Page: 1 of 8
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.02 Section: Prescription Drugs Effective Date: July 1, 2021 Subsection: Endocrine and Metabolic Drugs Original Policy Date: January 1, 2011 Subject: ART Drugs Page: 1 of 8 Last Review Date: June 17, 2021 ART Drugs Description Bravelle (urofollitropin) Cetrotide (cetrorelix) Clomid, Clomiphene Powder, Serophene (clomiphene citrate) Crinone, Endometrin, Progesterone in Oil, Progesterone Powder, Prometrium (progesterone) Follistim AQ (follitropin beta) Gonal-F, Gonal F RFF (follitropin alfa) Ganirelix (ganirelix) Menopur (menotropins) Milprosa (progesterone) Background Assisted Reproductive Technologies (ART) represent a group of non-coital manipulations and processes that manipulate ova and/or sperm to achieve a pregnancy. The most well-known examples are ovulation induction, intrauterine insemination and in-vitro fertilization. ART and infertility drugs used in conjunction with ART procedures or erectile or sexual dysfunction, weight loss, performance enhancement and anti-aging are not covered benefits. The diagnosis of hypogonadotropic hypogonadism is an off-label indication for these medications. A variety of drugs are used to manipulate the hypothalamic-pituitary-gonadal axis in order to induce ovulation in females known as controlled ovarian hyperstimulation (COH). Some of these pharmacologic agents are used for additional clinical care indications. Drugs Included in Infertility Drugs / ART Criteria • Antagon (ganirelix) – inhibition