DRUG and MEDICAL DEVICE HIGHLIGHTS Helping You Maintain and Improve Your Health 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fig. L COMPOSITIONS and METHODS to INHIBIT STEM CELL and PROGENITOR CELL BINDING to LYMPHOID TISSUE and for REGENERATING GERMINAL CENTERS in LYMPHATIC TISSUES
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ 23 February 2012 (23.02.2012) WO 2U12/U24519ft ft A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 31/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US201 1/048297 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 18 August 201 1 (18.08.201 1) NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/374,943 18 August 2010 (18.08.2010) US kind of regional protection available): ARIPO (BW, GH, 61/441,485 10 February 201 1 (10.02.201 1) US GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, 61/449,372 4 March 201 1 (04.03.201 1) US ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (72) Inventor; and EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, ΓΓ, LT, LU, (71) Applicant : DEISHER, Theresa [US/US]; 1420 Fifth LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Avenue, Seattle, WA 98101 (US). -

Acne Class Update
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-2596 Drug Class Update with New Drug Evaluation: Acne Drugs Date of Review: June 2020 Date of Last Review: November 2018 Dates of Literature Search: 08/03/2018 - 12/26/2019 Generic Name: Trifarotene Brand Name (Manufacturer): Aklief® (Galderma Laboratories, LP) Dossier Received: no Current Status of PDL Class: See Appendix 1. Purpose for Class Update: The acne class has had one new approval, trifarotene cream, and several new product formulations since it was last reviewed in 2018. The purpose of this update is to evaluate new comparative evidence for trifarotene cream for the treatment of acne vulgaris and any new data on comparative efficacy or harms in the acne class since the previous update. Acne conglobata, acne fulminans, and severe cystic acne are covered conditions under the Oregon Health Plan (OHP). Research Questions: 1. What is the comparative efficacy and effectiveness of treatments for severe acne (topical agents of adapalene, adapalene/benzoyl peroxide, tretinoin, tazarotene, benzoyl peroxide, salicylic acid, dapsone, azelaic acid, clindamycin, erythromycin, minocycline, sulfacetamide, trifarotene; oral systemic antibiotics of doxycycline, minocycline, tetracycline, azithromycin, erythromycin, clindamycin, trimethoprim, and sulfamethoxazole/trimethoprim; hormonal agents of oral contraceptives and spironolactone; and oral isotretinoin)? 2. What are the comparative harms of treatments for severe acne? 3. Are there subpopulations of patients in which a particular treatment for severe acne would be more effective or associated with less harm? Conclusions: There are no new high-quality clinical practice guidelines which have evaluated comparative efficacy and safety of treatments for acne vulgaris. -

Two Cases of Immediate Stent Fracture After Zotarolimus-Eluting
Case Report http://dx.doi.org/10.4070/kcj.2015.45.1.67 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal Two Cases of Immediate Stent Fracture after Zotarolimus-Eluting Stent Implantation Pil Hyung Lee, MD, Seung-Whan Lee, MD, Jong-Young Lee, MD, Young-Hak Kim, MD, Cheol Whan Lee, MD, Duk-Woo Park, MD, Seong-Wook Park, MD, and Seung-Jung Park, MD Department of Cardiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea Drug-eluting stent (DES) implantation is currently the standard treatment for various types of coronary artery disease. However, previous reports indicate that stent fractures, which usually occur after a period of time from the initial DES implantation, have increased during the DES era; stent fractures can contribute to unfavorable events such as in-stent restenosis and stent thrombosis. In our present report, we describe two cases of zotarolimus-eluting stent fracture: one that was detected six hours after implementation, and the other case that was detected immediately after deployment. Both anatomical and technical risk factors contributed to these unusual cases of imme- diate stent fracture. (Korean Circ J 2015;45(1):67-70) KEY WORDS: Drug-eluting stents; Percutaneous coronary intervention; Complications. Introduction eluting stent (ZES). Stent fractures have recently become an important concern in Cases the medical community due to their potential association with se- rious conditions such as in-stent restenosis (ISR) and stent throm- Case 1 bosis after drug-eluting stent (DES) implantation.1) Most currently A 62-year-old man, who had undergone cardiac transplantation reported stent fractures are found in lesions implanted with siroli- for advanced heart failure, was referred to our catheterization labo- mus-eluting stents (SES), likely due to the inherent platform material ratory for a regular surveillance coronary angiogram and concomi- and design of such stents.2) Furthermore, while the exact timing of tant intravascular ultrasound (IVUS) imaging. -

19F Henry Ford Health System Publication List – May 2021 This
19f Henry Ford Health System Publication List – May 2021 This bibliography aims to recognize the scholarly activity and provide ease of access to journal articles, meeting abstracts, book chapters, books and other works published by Henry Ford Health System personnel. Searches were conducted in PubMed, Embase, and Web of Science during the month, and then imported into EndNote for formatting. There are 144 unique citations listed this month, with 8 articles and 5 conference abstracts on COVID-19. Articles are listed first, followed by conference abstracts, books and book chapters, and a bibliography of publications on COVID-19. Because of various limitations, this does not represent an exhaustive list of all published works by Henry Ford Health System authors. Click the “Full Text” link to view the articles to which Sladen Library provides access. If the full- text of the article is not available, you may request it through ILLiad by clicking on “Request Article,” or calling us at (313) 916-2550. If you would like to be added to the monthly email distribution list to automatically receive a PDF of this bibliography, or you have any questions or comments, please contact [email protected]. If your published work has been missed, please use this form to notify us for inclusion on next month’s list. All articles and abstracts listed here are deposited into Scholarly Commons, the HFHS institutional repository. Jump to: Articles Administration Neurology Allergy and Immunology Neurosurgery Anesthesiology Obstetrics, Gynecology and Women’s Behavioral -

Related Kinases (VRK) Bound to Small-Molecule Inhibitors Identifies Different P-Loop Conformations
Structural characterization of human Vaccinia- Related Kinases (VRK) bound to small-molecule inhibitors identifies different P-loop conformations Rafael M. Couñago1,2*, Charles K. Allerston3, Pavel Savitsky3, Hatylas Azevedo4, Paulo H Godoi1,5, Carrow I. Wells6, Alessandra Mascarello4, Fernando H. de Souza Gama4, Katlin B. Massirer1,2, William J. Zuercher6, Cristiano R.W. Guimarães4 and Opher Gileadi1,3 1. Structural Genomics Consortium, Universidade Estadual de Campinas — UNICAMP, Campinas, SP, Brazil. 2. Centro de Biologia Molecular e Engenharia Genética, Universidade Estadual de Campinas, Campinas, SP, Brazil. 3. Structural Genomics Consortium and Target Discovery Institute, Nuffield Department of Clinical Medicine, University of Oxford, UK. 4. Aché Laboratórios Farmacêuticos SA, Guarulhos, SP, Brazil. 5. Department of Biochemistry and Tissue Biology, Institute of Biology, State University of Campinas, Campinas, Brazil. 6. Structural Genomics Consortium, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, NC, USA. *Correspondence to Rafael M. Couñago: [email protected] (RMC) Supplementary information 1 SUPPLEMENTARY METHODS PKIS results analyses - hierarchical cluster analysis (HCL) A hierarchical clustering (HCL) analysis was performed to group kinases based on their inhibition patterns across the compounds. The average distance clustering method was employed, using sample tree selection and sample leaf order optimization. The distance metric used was the Pearson correlation and the HCL analysis was performed in the TmeV software 1. SUPPLEMENTARY REFERENCES 1 Saeed, A. I. et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374-378, (2003). SUPPLEMENTARY FIGURES LEGENDS Supplementary Figure S1: Hierarchical clustering analysis of PKIS data. Hierarchical clustering analysis of PKIS data. -

2020 Research Annual Report Table of Contents
2020 RESEARCH ANNUAL REPORT TABLE OF CONTENTS PART I – INTERNAL MEDICINE DEPARTMENT ALLERGY AND IMMUNOLOGY ...................................................................................... 1 CARDIOLOGY/CARDIOVASCULAR RESEARCH…………………………………………..2 ENDOCRINOLOGY AND METABOLISM ........................................................................ 3 GASTROENTEROLOGY ................................................................................................ 5 HYPERTENSION AND VASCULAR RESEARCH……………………………………….…...6 PULMONARY……………………………………………………………………………………10 SLEEP MEDICINE………………………………………………………………………………10 GENERAL INTERNAL MEDICINE…………………………………………………….………12 HEMATOLOGY/ONCOLOGY………………………………………………………………….12 PART II – ALL OTHER CLINICAL DEPARTMENTS DERMATOLOGY ......................................................................................................... .14 EMERGENCY MEDICINE……………………………………………………………………..17 NEUROLOGY…………………………………………………………………………………...19 NEUROSURGERY……………………………………………………………………………… ORTHOPAEDICS/BONE & JOINT…………………………………………………………….31 OTOLARYNGOLOGY………………………………………………………………………… .36 PATHOLOGY ................................................................................................................ .36 PEDIATRICS…………………………………………………………………………………….37 PSYCHIATRY/BEHAVORIAL HEALTH………………………………………………………38 RADIATION ONCOLOGY ............................................................................................. 39 UROLOGY………………………………………………………………………………………40 WOMEN’S HEALTH……………………………………………………………………………42 i PART III POPULATION -

A20-73 Trifarotene (Acne Vulgaris) – Benefit Assessment According to §35A Social Code Book V1
IQWiG Reports – Commission No. A20-73 Trifarotene (acne vulgaris) – Benefit assessment according to §35a Social Code Book V1 Extract 1 Translation of Sections 2.1 to 2.5 of the dossier assessment Trifaroten (Acne vulgaris) – Nutzenbewertung gemäß § 35a SGB V (Version 1.0; Status: 12 November 2020). Please note: This translation is provided as a service by IQWiG to English-language readers. However, solely the German original text is absolutely authoritative and legally binding. Extract of dossier assessment A20-73 Version 1.0 Trifarotene (acne vulgaris) 12 November 2020 Publishing details Publisher Institute for Quality and Efficiency in Health Care Topic Trifarotene (acne vulgaris) – Benefit assessment according to §35a Social Code Book V Commissioning agency Federal Joint Committee Commission awarded on 13 August 2020 Internal Commission No. A20-73 Address of publisher Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen Im Mediapark 8 50670 Köln Germany Phone: +49 221 35685-0 Fax: +49 221 35685-1 E-mail: [email protected] Internet: www.iqwig.de Institute for Quality and Efficiency in Health Care (IQWiG) - i - Extract of dossier assessment A20-73 Version 1.0 Trifarotene (acne vulgaris) 12 November 2020 Medical and scientific advice . Prof. Dr. med. Dr. rer. nat. Enno Schmidt, University Hospital Schleswig-Holstein, Lübeck, Germany IQWiG thanks the medical and scientific advisor for his contribution to the dossier assessment. However, the advisor was not involved in the actual preparation of the dossier assessment. The responsibility for the contents of the dossier assessment lies solely with IQWiG. IQWiG employees involved in the dossier assessment . Anke Penno . Katharina Biester . Moritz Felsch . -

Aars Hot Topics Member Newsletter
AARS HOT TOPICS MEMBER NEWSLETTER American Acne and Rosacea Society 201 Claremont Avenue • Montclair, NJ 07042 (888) 744-DERM (3376) • [email protected] www.acneandrosacea.org Like Our YouTube Page Visit acneandrosacea.org to Become an AARS Member and TABLE OF CONTENTS Donate Now on acneandrosacea.org/donate AARS News Register Now for the AARS 9th Annual Scientific Symposium .................................... 2 Our Officers AARS BoD Member Emmy Graber invites you to earn free CME! ............................. 3 J. Mark Jackson, MD AARS President New Medical Research The effect of 577-nm pro-yellow laser on demodex density in patients with rosacea 4 Andrea Zaenglein, MD Aspirin alleviates skin inflammation and angiogenesis in rosacea ............................. 4 AARS President-Elect Efficacy and safety of intense pulsed light using a dual-band filter ............................ 4 Split-face comparative study of fractional Er:YAG laser ............................................. 5 Joshua Zeichner, MD Evaluation of biophysical skin parameters and hair changes ..................................... 5 AARS Treasurer Dermal delivery and follicular targeting of adapalene using PAMAM dendrimers ...... 6 Therapeutic effects of a new invasive pulsed-type bipolar radiofrequency ................ 6 Bethanee Schlosser, MD Efficacy and safety of a novel water-soluble herbal patch for acne vulgaris .............. 6 AARS Secretary A clinical study evaluating the efficacy of topical bakuchiol ........................................ 7 Tolerability and efficacy of clindamycin/tretinoin versus adapalene/benzoyl peroxide7 James Del Rosso, DO Photothermal therapy using gold nanoparticles for acne in Asian patients ................ 8 Director Development of a novel freeze-dried mulberry leaf extract-based transfersome gel . 8 The efficacy and safety of dual-frequency ultrasound for improving skin hydration ... 9 Emmy Graber, MD Director Clinical Reviews Jonathan Weiss, MD What the pediatric and adolescent gynecology clinician needs to know about acne . -
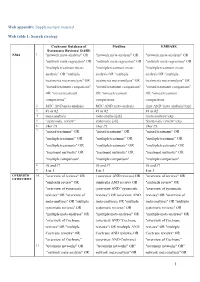
1 Web Appendix: Supplementary Material Web Table 1
Web appendix: Supplementary material Web table 1: Search strategy Cochrane Database of Medline EMBASE Systematic Reviews/ DARE NMA 1 "network meta-analysis" OR "network meta-analysis" OR "network meta-analysis" OR "network meta-regression" OR "network meta-regression" OR "network meta-regression" OR "multiple treatment meta- "multiple treatment meta- "multiple treatment meta- analysis” OR "multiple analysis OR "multiple analysis OR "multiple treatments meta-analysis" OR treatments meta-analysis" OR treatments meta-analysis" OR "mixed treatment comparison" "mixed treatment comparison" "mixed treatment comparison" OR "mixed treatment OR "mixed treatment OR "mixed treatment comparisons” comparisons comparisons 2 MTC AND meta-analysis MTC AND meta-analysis (mtc AND 'meta analysis'/exp) 3 #1 or #2 #1 or #2 #1 or #2 4 meta-analysis meta-analysis[sb] 'meta analysis'/exp 5 “systematic review” systematic [sb] 'Systematic review '/exp 6 #4or #5 #4or #5 #4or #5 7 "mixed treatment" OR "mixed treatment" OR "mixed treatment" OR "multiple treatment" OR "multiple treatment" OR "multiple treatment" OR "multiple treatments" OR "multiple treatments" OR "multiple treatments" OR "treatment networks" OR "treatment networks" OR "treatment networks" OR "multiple comparison" "multiple comparison" "multiple comparison" 8 #6 and #7 #6 and #7 #6 and #7 9 8 or 3 8 or 3 8 or 3 OVERVIEW 10 "overview of reviews" OR ( overview AND reviews) OR "overview of reviews" OR OF REVIEWS "umbrella review" OR (umbrella AND review) OR "umbrella review" OR "overview of systematic -

That Are Not Lielu Uutuullittu
THAT ARE NOT LIELUUS009987356B2 UUTUULLITTU (12 ) United States Patent ( 10 ) Patent No. : US 9 ,987 , 356 B2 Reimann et al. ( 45) Date of Patent : Jun . 5 , 2018 (54 ) ANTI -CD40 ANTIBODIES AND METHODS 762 A 12 / 1997 Queen et al. 5 , 801, 227 A 9 / 1998 Fanslow , III et al. OF ADMINISTERING THEREOF 5 , 874 ,082 A 2 / 1999 de Boer 6 ,004 , 552 A 12 / 1999 de Boer et al. ( 75 ) Inventors: Keith A . Reimann , Marblehead , MA 6 ,051 ,228 A 4 / 2000 Aruffo et al. (US ) ; Rijian Wang , Saugus, MA (US ) ; 6 ,054 ,297 A 4 / 2000 Carter et al . Christian P . Larsen , Atlanta , GA (US ) 6 ,056 , 959 A 5 /2000 de Boer et al. 6 , 132 , 978 A 10 /2000 Gelfand et al. 6 , 280 , 957 B18 / 2001 Sayegh et al. ( 73) Assignees : Beth Israel Deaconess Medical 6 , 312 ,693 B1 11/ 2001 Aruffo et al. Center , Inc ., Boston , MA (US ) ; Emory 6 , 315 , 998 B111 / 2001 de Boer et al. University , Atlanta , GA (US ) 6 , 413 ,514 B1 7 / 2002 Aruffo et al. 6 , 632, 927 B2 10 /2003 Adair et al. ( * ) Notice : Subject to any disclaimer , the term of this 7 ,063 , 845 B2 6 / 2006 Mikayama et al. patent is extended or adjusted under 35 7 , 193 , 064 B2 3 / 2007 Mikayama et al. 7 , 288 , 251 B2 10 / 2007 Bedian et al . U . S . C . 154 ( b ) by 464 days . 7 , 361 , 345 B2 4 /2008 de Boer et al. 7 , 445 , 780 B2 11/ 2008 Chu et al. (21 ) Appl . No. -

WHO Drug Information Vol. 20, No. 3, 2006
WHO DRUG INFORMATION VOLUME 20• NUMBER 3 • 2006 RECOMMENDED INN LIST 56 INTERNATIONAL NONPROPRIETARY NAMES FOR PHARMACEUTICAL SUBSTANCES WORLD HEALTH ORGANIZATION • GENEVA WHO Drug Information Vol 20, No. 3, 2006 World Health Organization WHO Drug Information Contents Biomedicines and Vaccines Counterfeit taskforce launched 192 Fake artesunate warning sheet 193 Monoclonal antibodies: a special Resolution on counterfeiting 193 regulatory challenge? 165 Improving world health through regulation of biologicals 173 Regulatory Action and News Influenza virus vaccines for 2006–2007 Safety and Efficacy Issues northern hemisphere 196 Trastuzumab approved for primary Global progress in monitoring immuniza- breast cancer 196 tion adverse events 180 Emergency contraception over-the- Intracranial haemorrhage in patients counter 197 receiving tipranavir 180 Ocular Fusarium infections: ReNu Infliximab: hepatosplenic T cell MoistureLoc® voluntary withdrawal 197 lymphoma 181 Saquinavir: withdrawal of soft gel Lamotrigine: increased risk of non- capsule Fortovase® 197 syndromic oral clefts 181 Latest list of prequalified products and Biphosphonates: osteonecrosis of manufacturers 198 the jaw 181 Clopidogrel: new medical use 199 Enoxaparin dosage in chronic kidney Dronedarone: withdrawal of marketing disease 182 authorization 199 Terbinafine and life-threatening blood dyscrasias 183 Adverse reactions in children: why Recent Publications, report? 183 Hepatitis B reactivation and anti-TNF- Information and Events alpha agents 185 MSF issues ninth antiretroviral -

213433Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 213433Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review NDA/BLA Multidisciplinary Review and Evaluation: NDA 213433 WINLEVI (clascoterone) cream, 1% NDA Multidisciplinary Review and Evaluation Application Type NME- In “The Program” Application Number(s) NDA 213433 Priority or Standard Standard Submit Date(s) August 19, 2019 Received Date(s) August 27, 2019 PDUFA Goal Date August 27, 2020 Division/Office Shari Targum, Deputy Director, Division of Dermatology and Dentistry Julie Beitz, Director Office of Immunology and Inflammation Review Completion Date Established/Proper Name Clascoterone cream, 1% (Proposed) Trade Name WINLEVI Pharmacologic Class NME Code Name N/A Applicant Cassiopea SpA Dosage Form Topical cream Applicant proposed Dosing Apply a thin layer (approximately 1 gram) to affected area Regimen twice daily (morning and evening) Applicant Proposed For the treatment of acne vulgaris in patients 9 years of age Indication(s)/Population(s) and older Applicant Proposed SNOMED CT Indication Disease Term for Each Proposed Indication Recommendation on Regulatory Action Approval Recommended For the treatment of acne vulgaris in patients 12 years of age Indication(s)/Population(s) and older (if applicable) Recommended SNOMED CT Indication Disease Term for Each Indication (if applicable) Recommended Dosing Apply a thin layer (approximately 1 gram) to affected area Regimen twice daily (morning and