Follicle Stimulating Hormone (FSH) Gonadotropins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -

Human Chorionic Gonadotropin (HCG), a Polypeptide Hormone Produced by the Human
45792G/Revised: April 2011 CHORIONIC GONADOTROPIN FOR INJECTION, USP DESCRIPTION: Human chorionic gonadotropin (HCG), a polypeptide hormone produced by the human placenta, is composed of an alpha and a beta sub-unit. The alpha sub-unit is essentially identical to the alpha sub-units of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha sub-unit of human thyroid-stimulating hormone (TSH). The beta sub-units of these hormones differ in amino acid sequence. Chorionic gonadotropin is obtained from the human pregnancy urine. It is standardized by a biological assay procedure. Chorionic Gonadotropin for Injection, USP is available in multiple dose vials containing 10,000 USP Units with accompanying Bacteriostatic Water for Injection for reconstitution. When reconstituted with 10 mL of the accompanying diluent each vial contains: Chorionic gonadotropin 10,000 Units Mannitol 100 mg Benzyl alcohol 0.9% Water for Injection q.s. Buffered with dibasic sodium phosphate and monobasic sodium phosphate. Hydrochloric acid and/or sodium hydroxide may have been used for pH adjustment (6.0 Reference ID: 2933198 8.0). Nitrogen gas is used in the freeze drying process. CLINICAL PHARMACOLOGY: The action of HCG is virtually identical to that of pituitary LH, although HCG appears to have a small degree of FSH activity as well. It stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce androgens and the corpus luteum of the ovary to produce progesterone. Androgen stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. -

Gonadotropin and Testosterone Measurements After
Pediat. Res. 10: 46-51 (1976) Estradiol puberty estrogen sexual differentiation gonadotropins testosterone maturation Gonadotropin and Testosterone Measurements after Estrogen Administration to Adult Men, Prepubertal and Pubertal Boys, and Men with Hypogonadotropism: Evidence for Maturation of Positive Feedback in the Male HOWARD E. KULIN"" AND EDWARD 0. REITER Reproduction Research Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA Extract MATERIALS AND METHODS Nineteen male subjects were given five daily injections of SUBJECTS 17~-estradiol and circulating levels of estradiol (E 2 ), testosterone (T), and gonadotropins were determined by radioimmunoassay Seven normal, adult men (ages 20-22) and one male (age 21) before, during, and after the steroid course. Peak levels of E 2 with the syndrome of vanishing testes (I) were hospitalized for attained during the 5 days of treatment ranged from 173-577 pg/ml. study at the Clinical Center of the National Institutes of Health. Four of seven normal adult men and one castrate man demonstrated Each patient was given five daily intramuscular injections of IO or suppression of follicle-stimulating hormone ( FSH) and luteinizing 15 ,ug/kg body weight of 17~-estradiol (E2 ) (20) and blood samples hormone ( LH) with a subsequent rise in LH ( positive feedback) were obtained every 12-24 hr before, during, and after the estrogen while E 2 levels remained elevated. A rise in T was associated with course (see Table I). the LH increment in the four normal men. Nine pre-, early, or Nine endocrinologically normal boys between the ages of 7 and midpubertal boys and two men with hypogonadotropic hypogo 18 were studied in the course of evaluation for short stature, nadism displayed only gonadotropin suppression after E 2 adminis precocious puberty, or delayed adolescence. -
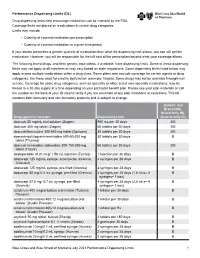
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Chorionic Gonadotropin Human (C0684)
Chorionic gonadotropin human Product Number C 0684 Storage Temperature -0 °C Product Description When hCG was used in combination with recombinant CAS Number: 9002-61-3 interferon-γ, there was a significant cooperative pI = 2.951 induction of nitric oxide synthesis (iNOS) in a dose- Extinction Coefficient: E1% = 3.88 (278nm)2 dependent manner in mouse peritoneal macrophages Synonym: Choriogonin, hCG suggesting that hCG may provide a second signal for synergistic induction of NO synthesis.9 The molecular weight is approximately 37.9 kDa (with approximately 31% carbohydrate by weight). The Precautions and Disclaimer theoretical molecular weight is 37.9 kDa based on the For Laboratory Use Only. Not for drug, household or native form, which contains 2 subunits. The α subunit other uses. has a molecular weight of 14.9 kDa of which approximately 10.2 kDa is for the polypeptide and Preparation Instructions approximately 4.7 kDa for the carbohydrate. The hCG is soluble in water and aqueous buffers such β subunit has a molecular weight of 23 kDa of which phosphate buffer. hCG is also soluble in aqueous approximately 16.0 kDa is for the polypeptide and glycerol and glycols and is insoluble in ethanol.1 approximately 7.0 kDa is for the carbohydrate.3,4,5 Solutions should be sterile filtered and not autoclaved. Product Number C 0684 is sterile filtered and contains Storage/Stability approximately 1,000 I.U. per vial. Dilute aqueous solutions undergo rapid loss of activity when stored frozen, or heated, or if excess acid or hCG is a glycoprotein hormone produced by the base is added. -

Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry
Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be submitted within 90 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit electronic comments to https://www.regulations.gov. Submit written comments to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. For questions regarding this draft document, contact Elaine Chang at 240-402-2628. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) July 2019 Clinical/Medical Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353; Email: [email protected] https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation -

WSAVA List of Essential Medicines for Cats and Dogs
The World Small Animal Veterinary Association (WSAVA) List of Essential Medicines for Cats and Dogs Version 1; January 20th, 2020 Members of the WSAVA Therapeutic Guidelines Group (TGG) Steagall PV, Pelligand L, Page SW, Bourgeois M, Weese S, Manigot G, Dublin D, Ferreira JP, Guardabassi L © 2020 WSAVA All Rights Reserved Contents Background ................................................................................................................................... 2 Definition ...................................................................................................................................... 2 Using the List of Essential Medicines ............................................................................................ 2 Criteria for selection of essential medicines ................................................................................. 3 Anaesthetic, analgesic, sedative and emergency drugs ............................................................... 4 Antimicrobial drugs ....................................................................................................................... 7 Antibacterial and antiprotozoal drugs ....................................................................................... 7 Systemic administration ........................................................................................................ 7 Topical administration ........................................................................................................... 9 Antifungal drugs ..................................................................................................................... -

The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men
ORIGINAL ARTICLE Endocrine Research The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men M. Y. Roth, S. T. Page, K. Lin, B. D. Anawalt, A. M. Matsumoto, B. Marck, W. J. Bremner, and J. K. Amory Downloaded from https://academic.oup.com/jcem/article/96/4/1175/2720870 by guest on 02 October 2021 Departments of Internal Medicine (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.) and Obstetrics and Gynecology (K.L.) and Center for Research in Reproduction and Contraception (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.), University of Washington, Seattle, Washington 91895; and Geriatric Research (B.M.), Education and Clinical Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington 98105 Introduction: Concentrations of intratesticular (IT) testosterone (T) are known to be 100–200 times those of serum T; however, the IT concentrations of T’s precursors, their testicular to serum gra- dients, gonadotropin dependence, and response to stimulation with human chorionic gonado- tropin (hCG) have not been studied in detail. We hypothesized that serum and IT androstenedione (ADD) and IT dehydroepiandrosterone (DHEA) would be significantly suppressed by the adminis- tration of a GnRH antagonist and increased when stimulated by hCG, without a similar suppression of serum DHEA. Methods: We suppressed gonadotropins in 23 normal men with the GnRH antagonist acyline and randomly assigned them to one of four doses of hCG, 0, 15, 60, or 125 IU sc every other day for 10 d. -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage -

Gonadotropin Releasing Hormone Test
Paediatric & Adolescent Endocrinology Yorkshire Regional Centre Leeds Children’s Hospital Gonadotrophin Releasing Hormone (GnRH) test http://www.pathology.leedsth.nhs.uk/dnn_bilm/Investigationprotocols/Pituitaryprotocols/GnRHTest.aspx Indication To diagnose hypothalamic-pituitary disease in precocious and delayed puberty in both sexes in those children with low basal gonadotrophins. Contra-indications This test may be performed simultaneously with TRH or glucagon as part of triple pituitary test. Principle GnRH (gonadotrophin releasing hormone) is a decapeptide secreted by the hypothalamus which stimulates the production and secretion of LH and FSH by the anterior pituitary. Side effects GnRH may rarely cause nausea, headache and abdominal pain. Preparation No specific patient preparation is required. Requirements 3 plain tubes Drug administration: GnRH (Gonadorelin) 2.5 microgram/kg to a maximum of 100 microgram Procedure take 2 mL blood for LH & FSH and testosterone (males) and oestradiol time 0 min (females) immediately give GnRH IV as a bolus (dose as above) time 20 min take 2 mL blood for LH & FSH time 60 min take 2 mL blood for LH & FSH Interpretation 1. Normal basal reference values in prepubertal children are: LH < 2.0 IU/L FSH < 2.0 IU/L 2. Following GnRH, the response may be considered normal if the basal values are in the reference range and there is at least a doubling at 20 min for LH and FSH. The response varies throughout the menstrual cycle: early (D4) < late follicular (D11) = "luteal" (D21), max response occurs at the mid- cycle (D14). 3. An exaggerated response is seen in primary & secondary gonadal failure. 4.