ART / Infertility Drugs / Gender Dysphoria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -

The ART of Assisted Reproductive Technology
Infertility • THEME The ART of assisted reproductive technology BACKGROUND One in six Australian couples of The Australian Institute of Health and Welfare's reproductive age experience difficulties in National Perinatal Statistical Unit's annual report Jeffrey Persson, conceiving a child. Once a couple has been Assisted reproductive technology in Australia and New MD, FRANZCOG, CREI, is Clinical appropriately assessed, assisted reproductive Zealand 2002, shows that babies born in 2002 using Director (City), technology (ART) techniques can be used to assisted reproductive technology (ART) had longer IVFAustralia. overcome problems with ovulation, tubal patency, gestational ages, higher birth weights and fewer peri- [email protected] male fertility or unexplained infertility. natal deaths than their counterparts of 2 years OBJECTIVE This article discusses the range of previously.1 ART techniques available to subfertile couples. This is the first year national data have been avail- able on the age of women (and their partners) using DISCUSSION Intracytoplasmic sperm injection is now the most common form of ART used in ART. The average age of women undergoing treat- Australia, being used in almost half of all fresh ment in 2002 was 35.2 years, and the average cycles, with in vitro fertilisation being used in age of partners was 37.6 years.1 Age remains the approximately one-third of all fresh cycles. most significant issue affecting a couple's chance of conceiving and carrying a pregnancy to full term. Women need to understand the impact age has on their chance of becoming pregnant. Once a couple has been assessed and investigations suggest subfer- tility, the treatment options depend on the underlying cause (Table 1). -

Obesity and Reproduction: a Committee Opinion
Obesity and reproduction: a committee opinion Practice Committee of the American Society for Reproductive Medicine American Society for Reproductive Medicine, Birmingham, Alabama The purpose of this ASRM Practice Committee report is to provide clinicians with principles and strategies for the evaluation and treatment of couples with infertility associated with obesity. This revised document replaces the Practice Committee document titled, ‘‘Obesity and reproduction: an educational bulletin,’’ last published in 2008 (Fertil Steril 2008;90:S21–9). (Fertil SterilÒ 2015;104:1116–26. Ó2015 Use your smartphone by American Society for Reproductive Medicine.) to scan this QR code Earn online CME credit related to this document at www.asrm.org/elearn and connect to the discussion forum for Discuss: You can discuss this article with its authors and with other ASRM members at http:// this article now.* fertstertforum.com/asrmpraccom-obesity-reproduction/ * Download a free QR code scanner by searching for “QR scanner” in your smartphone’s app store or app marketplace. he prevalence of obesity as a exceed $200 billion (7). This populations have a genetically higher worldwide epidemic has underestimates the economic burden percent body fat than Caucasians, T increased dramatically over the of obesity, since maternal morbidity resulting in greater risks of developing past two decades. In the United States and adverse perinatal outcomes add diabetes and CVD at a lower BMI of alone, almost two thirds of women additional costs. The problem of obesity 23–25 kg/m2 (12). and three fourths of men are overweight is also exacerbated by only one third of Known associations with metabolic or obese, as are nearly 50% of women of obese patients receiving advice from disease and death from CVD include reproductive age and 17% of their health-care providers regarding weight BMI (J-shaped association), increased children ages 2–19 years (1–3). -
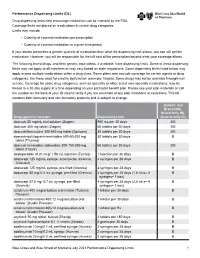
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

5.01.610 Pharmacologic Treatment of Infertility
PHARMACY / MEDICAL POLICY – 5.01.610 Pharmacologic Treatment of Infertility Effective Date: Feb. 1, 2021 RELATED MEDICAL POLICIES: Last Revised: Jan. 21, 2021 4.02.503 Infertility and Reproductive Services Replaces: N/A Select a hyperlink below to be directed to that section. POLICY CRITERIA | DOCUMENTATION REQUIREMENTS | CODING RELATED INFORMATION | EVIDENCE REVIEW | REFERENCES | HISTORY ∞ Clicking this icon returns you to the hyperlinks menu above. Introduction Infertility is a problem or problems with the reproductive system that affects the ability to conceive. Different types of reproductive problems affect men and women, but the end result is the inability to conceive or complete a pregnancy. There are many reasons for infertility and drug options vary depending on the cause of infertility and type of infertility treatment required. Even though drug treatment exists, it does not mean it is covered; the member’s contract determines this. This policy describes when infertility drugs may be considered medically necessary if covered by the member’s contract. Note: The Introduction section is for your general knowledge and is not to be taken as policy coverage criteria. The rest of the policy uses specific words and concepts familiar to medical professionals. It is intended for providers. A provider can be a person, such as a doctor, nurse, psychologist, or dentist. A provider also can be a place where medical care is given, like a hospital, clinic, or lab. This policy informs them about when a service may be covered. Policy Coverage -

Ovulation Induction/Intrauterine Insemination” Works
UW MEDICINE | PATIENT EDUCATION | | Ovulation Induction/Intrauterine | Insemination | What to expect This handout is for patients at University Reproductive Care (URC). It explains how a fertility treatment called “ovulation induction/intrauterine insemination” works. How does this fertility treatment work? This treatment increases the chance of pregnancy: • For women who do not ovulate on their own • For women who have a low number of eggs • For men who have mildly low sperm counts or motility • When the cause of infertility is unknown • For women who use donor Please talk with a provider at sperm University Reproductive Care if you Each step of this fertility have any questions about this fertility treatment is important: treatment. • Clomiphene citrate (Clomid) and letrozole (Femara) are oral medicines that help eggs grow and mature to prepare for ovulation, the release of a mature egg from the ovarian follicle (egg sac). You will take one of these oral medicines for at least 5 days. • You will have a pelvic ultrasound in the middle part of the cycle. It is used to determine when your follicle/follicles are ready for an ovulation trigger injection. • The ovulation trigger injection (human chorionic gonadotropin/hCG, or Lupron) helps the egg mature and determines the time of your intrauterine insemination. • Intrauterine insemination places the most motile (moving) sperm as close as possible to the egg(s) at the time when fertilization is most likely. This helps increase the chance of pregnancy. _______________________________________________________________________________________ ______ Page 1 of 2 | Ovulation Induction/Intrauterine Insemination University Reproductive Care | Box 356460 4245 Roosevelt Way N.E., Seattle, WA 98105 | 206.598.4225 What are the possible risks from this treatment? The risks linked to the oral ovulation induction medicines include: • Having twins: Less than 5% of women treated at URC (fewer than 5 out of 100 women) who become pregnant using clomiphene citrate have twins. -

Ovulation Induction Information Packet Pre-Treatment Recommendations
Ovulation Induction Information Packet Ovulation inducing medications help infertile women achieve a pregnancy by stimulating the development of multiple eggs within the ovaries. The medications are often used in conjunction with intrauterine inseminations with a washed sperm sample. This document explains ovulation inducing medications and intrauterine insemination treatment. Pre-Treatment Recommendations Patients should avoid any activity, medication, or behaviors that would reduce their chances of conception or increase the risk to the baby. Below are listed recommendations for all women/couples attempting pregnancy: Women should take a prenatal vitamin on a daily basis. This vitamin should contain folic acid which reduces the chance of giving birth to a child with a neural tube defect (e.g. spina bifida). Unless contraindicated, women should also take aspirin 81mg on a daily basis Smoking must be avoided before and during treatment. It is also contraindicated during pregnancy. Recreational drugs are absolutely contraindicated. Avoid ingestion of adult strength aspirin (325mg). Aspirin-like products (e.g. Motrin®, Advil®, Anaprox®, Naprosyn®, Aleve®, etc.) should be avoided during ovulation and Timed Intercourse (TIC)/Intrauterine Insemination (IUI) time. Tylenol® is a suitable alternative. After ovulation, Motrin® may be used as well. The use of alcohol should be eliminated, as it is contraindicated during pregnancy. The use of all prescription and over-the-counter medications should be discussed with a physician before starting a treatment cycle. Women should continue to get yearly Pap tests done with their primary care providers. Men should take a multivitamin and antioxidant (antioxidant supplements usually include vitamins E and C, beta carotene and selenium) on a daily basis. -

Specialty Guideline Management
SPECIALTY GUIDELINE MANAGEMENT North Carolina State Health Plan: Fertility Agents PROGRAM RATIONALE Client Requested: The intent of the criteria is to ensure that patients follow selection elements established by North Carolina State Health Plan’s Commercial Prior Authorization Approval policy. PRIOR AUTHORIZATION CRITERIA1 • Coverage is provided for female infertility treatment and in males for non-infertility indications. • Coverage is NOT provided for patients using fertility medication in conjunction with any type of Artificial Reproductive Technology (ART) procedure. ART procedures include In Vitro Fertilization (IVF), Gamete Intrafallopian Transfer (GIFT), Zygote Intrafallopian Transfer (ZIFT) and Intrauterine or Artificial Insemination. COVERED FERTILITY AGENTS* Medication Generic Name Covered Indications Gonadotropins Follicle Stimulating Hormone (FSH) Bravelle† urofollitropin . Ovulation induction Follistim AQ follitropin beta . Ovulation induction . Hypogonadotropic hypogonadism in males Gonal-F†/ follitropin alfa . Ovulation induction Gonal-F RFF Pen† . Hypogonadotropic hypogonadism in males (Gonal-F only) Human Chorionic Gonadotropin (hCG) Novarel, Pregnyl, chorionic gonadotropin . Ovulation induction hcG (generic) . Selected cases of hypogonadotropic hypogonadism in males (ie, hypogonadism secondary to a pituitary deficiency) . Prepubertal cryptorchidism Ovidrel choriogonadotropin alfa . Ovulation induction Human Menopausal Gonadotropin (hMG) Menopur menotropin . Ovulation induction Gonadotropin Releasing Hormone (GnRH) Analogs -

Editorial Robert L
Editorial Robert l. Barbieri, mD Editor in Chief ClOmIPhEnE failuRE? Try adding dexamethasone to your clomiphene infertility regimen Clomiphene-resistant women often ovulate, and then become pregnant, when treated with a combination of clomiphene and dexamethasone CASE Infertility Dx: PCOS, For women who have PCOS and work-up shows severely abnormal anovulation anovulatory infertility, approaches to semen parameters or bilateral tubal You have been treating a 33-year-old ovulation induction include: disease, consultation with a fertility G0P0 woman who has polycystic ovary • weight loss specialist, and IVF, might be a more syndrome (PCOS) and anovulatory in- • clomiphene appropriate course than undertaking fertility with clomiphene citrate for three • metformin ovulation induction with clomiphene. cycles, at escalating doses of 50 mg, • follicle-stimulating hormone (FSH) Further work-up of anovulatory 100 mg, and 150 mg daily. She has not injection infertility. First obtain measure- ovulated, however, as determined by • laparoscopic ovarian drilling ments of serum thyroid-stimulating appropriately timed serum progester- • in vitro fertilization (IVF). hormone (TSH), follicle-stimulating one measurement. Women who fail to ovulate at hormone (FSH), and prolactin; ab- What is your next step? standard dosages of clomiphene are normalities of these hormones might labeled “clomiphene-resistant.” A contraindicate clomiphene for ovu- novulation is a common consensus panel of expert fertility spe- lation induction. cause of infertility; approxi- cialists recommended that, for such Next, measurement of total se- A mately 70% of cases of anovu- women, the most appropriate next rum testosterone and dehydroepi- latory infertility are caused by PCOS. steps in treatment include FSH injec- androsterone sulfate (DHEAS) might tion or laparoscopic ovarian drilling.1 be useful to determine if your patient’s For many women, however, those op- clomiphene resistance is caused by Instant Poll tions are prohibitively expensive. -

ART Drugs Page: 1 of 8
Federal Employee Program® 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.02 Section: Prescription Drugs Effective Date: July 1, 2021 Subsection: Endocrine and Metabolic Drugs Original Policy Date: January 1, 2011 Subject: ART Drugs Page: 1 of 8 Last Review Date: June 17, 2021 ART Drugs Description Bravelle (urofollitropin) Cetrotide (cetrorelix) Clomid, Clomiphene Powder, Serophene (clomiphene citrate) Crinone, Endometrin, Progesterone in Oil, Progesterone Powder, Prometrium (progesterone) Follistim AQ (follitropin beta) Gonal-F, Gonal F RFF (follitropin alfa) Ganirelix (ganirelix) Menopur (menotropins) Milprosa (progesterone) Background Assisted Reproductive Technologies (ART) represent a group of non-coital manipulations and processes that manipulate ova and/or sperm to achieve a pregnancy. The most well-known examples are ovulation induction, intrauterine insemination and in-vitro fertilization. ART and infertility drugs used in conjunction with ART procedures or erectile or sexual dysfunction, weight loss, performance enhancement and anti-aging are not covered benefits. The diagnosis of hypogonadotropic hypogonadism is an off-label indication for these medications. A variety of drugs are used to manipulate the hypothalamic-pituitary-gonadal axis in order to induce ovulation in females known as controlled ovarian hyperstimulation (COH). Some of these pharmacologic agents are used for additional clinical care indications. Drugs Included in Infertility Drugs / ART Criteria • Antagon (ganirelix) – inhibition