Duodenal Carcinomas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendiceal GCC and LAMN Navigating the Alphabet Soup in the Appendix
2018 Park City AP Update Appendiceal GCC and LAMN Navigating the Alphabet Soup in the Appendix Sanjay Kakar, MD University of California, San Francisco Appendiceal tumors Low grade appendiceal mucinous neoplasm • Peritoneal spread, chemotherapy • But not called ‘adenocarcinoma’ Goblet cell carcinoid • Not a neuroendocrine tumor • Staged and treated like adenocarcinoma • But called ‘carcinoid’ Outline • Appendiceal LAMN • Peritoneal involvement by mucinous neoplasms • Goblet cell carcinoid -Terminology -Grading and staging -Important elements for reporting LAMN WHO 2010: Low grade carcinoma • Low grade • ‘Pushing invasion’ LAMN vs. adenoma LAMN Appendiceal adenoma Low grade cytologic atypia Low grade cytologic atypia At minimum, muscularis Muscularis mucosa is mucosa is obliterated intact Can extend through the Confined to lumen wall Appendiceal adenoma: intact muscularis mucosa LAMN: Pushing invasion, obliteration of m mucosa LAMN vs adenocarcinoma LAMN Mucinous adenocarcinoma Low grade High grade Pushing invasion Destructive invasion -No desmoplasia or -Complex growth pattern destructive invasion -Angulated infiltrative glands or single cells -Desmoplasia -Tumor cells floating in mucin WHO 2010 Davison, Mod Pathol 2014 Carr, AJSP 2016 Complex growth pattern Complex growth pattern Angulated infiltrative glands, desmoplasia Tumor cells in extracellular mucin Few floating cells common in LAMN Few floating cells common in LAMN Implications of diagnosis LAMN Mucinous adenocarcinoma LN metastasis Rare Common Hematogenous Rare Can occur spread -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Associated Lymphoid Tissue in Chickens and Turkeys Andrew Stephen Fix Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1990 The trs ucture and function of conjunctiva- associated lymphoid tissue in chickens and turkeys Andrew Stephen Fix Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Animal Sciences Commons, and the Veterinary Medicine Commons Recommended Citation Fix, Andrew Stephen, "The trs ucture and function of conjunctiva-associated lymphoid tissue in chickens and turkeys " (1990). Retrospective Theses and Dissertations. 9496. https://lib.dr.iastate.edu/rtd/9496 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. Hie quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-Tailed Opossum (Monodelphis Domestica) and North American Opossum (Didelphis Virginiana)
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2001 Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana) Lee Anne Cope University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Animal Sciences Commons Recommended Citation Cope, Lee Anne, "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana). " PhD diss., University of Tennessee, 2001. https://trace.tennessee.edu/utk_graddiss/2046 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Lee Anne Cope entitled "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana)." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Animal Science. Robert W. Henry, Major Professor We have read this dissertation and recommend its acceptance: Dr. R.B. Reed, Dr. C. Mendis-Handagama, Dr. J. Schumacher, Dr. S.E. Orosz Accepted for the Council: Carolyn R. -

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

TUMOR and STAGING DATA Primary Site Code
SECTION IV - TUMOR and STAGING DATA Primary Site Code NAACCR Version 11.1 field "Primary Site", Item 400, columns 291-294 It is unclear how the 2007 MP/H rules may alter rules for assigning the best Primary Site Formatted: Left Code to each primary. Continue to use the following rules until new rules are issued. Enter the code for the site of origin from the Topography section of ICD-O-3. [Note that ICD-O-2 code C14.1, laryngopharynx, should not be used for diagnoses made on or after January 1, 1995. "Laryngopharynx" became an equivalent term under C13.9 (hypopharynx, NOS) as of this diagnosis date. Code C14.1 is not an ICD-O-3 code.] Enter the site code that matches the narrative primary site indicated in the medical record, or the site code most appropriate for the case. Site codes are found in ICD-O-3's Numerical Lists - Topography section (pages 45-65) and in its Alphabetic Index (pages 105-218). In ICD-O-3 primary site codes consist of the letter "C" followed by two digits, a decimal point, and a third digit. "C" should be entered but the decimal point should not be entered. Example: The primary site is "cardia of stomach". Look this up in the Alphabetic Index of ICD-O-3 under "stomach" or "cardia", and the corresponding code "C16.0" is found. Enter C160. Most sites include a third digit of "8" to be used for single tumors that overlap the boundaries of two or more anatomically contiguous subsites and whose exact point of origin cannot be determined, unless the combination of sites is specifically indexed elsewhere. -

2018 SEER Solid Tumor Manual
4/6/2018 Eight Groups are Revised for 2018 Head & Neck Colon (includes rectosigmoid and rectum for cases diagnosed 1/1/2018 forward) 2018 SEER Lung (2018 Draft not yet available) Breast Solid Tumor Manual Kidney Urinary Sites (2018 Draft not yet available) 2018 KCR SPRING TRAINING Non‐malignant CNS (2018 Draft not yet available) Malignant CNS and Peripheral Nerves (2018 Draft not yet available) 2019 Changes for other two Groups Solid Tumor Rules What we will cover: ◦ Overview of General rules Cutaneous melanoma (minor revisions and draft available now) • Cutaneous melanoma site rules will be revised for 2019 implementation to incorporate ◦ New Head and Neck rules information from the new WHO 4th Ed Tumors of Skin scheduled to be released in 2018. ◦ New Colorectal rules ◦ New Breast rules Other sites (minor revisions and draft available now) ◦ New Kidney rules • Primary sites excluded are: •Rectosigmoid and rectum which are included in 2018 Colon rules. •Peripheral nerves which are included in 2018 Malignant Brain rules. •Other sites rules will be revised for 2019 implementation. The Solid Tumor Task Force has identified Remember: These are currently in draft form and may change slightly in the final version! the need to expand the rules to include GYN, soft tissue, thyroid and other site‐specific solid tumors. NEW! Code subtypes/variants when definitively described (with no modifiers) General Instructions Example: Well ‐differentiated neuroendocrine tumor 8240. Do not code a histology (*including subtypes or variants) when described as below: The 2018 solid tumor rules replace all previous MP/H rules, but they are effective for diagnoses on or after 1/1/2018. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

New Developments in Goblet Cell Mucus Secretion and Function
REVIEW nature publishing group New developments in goblet cell mucus secretion and function GMH Birchenough1, MEV Johansson1, JK Gustafsson1, JH Bergstro¨m1 and GC Hansson1 Goblet cells and their main secretory product, mucus, have long been poorly appreciated; however, recent discoveries have changed this and placed these cells at the center stage of our understanding of mucosal biology and the immunology of the intestinal tract. The mucus system differs substantially between the small and large intestine, although it is built around MUC2 mucin polymers in both cases. Furthermore, that goblet cells and the regulation of their secretion also differ between these two parts of the intestine is of fundamental importance for a better understanding of mucosal immunology. There are several types of goblet cell that can be delineated based on their location and function. The surface colonic goblet cells secrete continuously to maintain the inner mucus layer, whereas goblet cells of the colonic and small intestinal crypts secrete upon stimulation, for example, after endocytosis or in response to acetyl choline. However, despite much progress in recent years, our understanding of goblet cell function and regulation is still in its infancy. THE INTESTINE system of mucus covering the epithelium. There is a The gastrointestinal tract is a remarkable organ. Not only can it two-layered mucus system in the stomach and colon and a digest most of our food into small components, but it is also single-layered mucus in the small intestine.5 The mucus layers filled with kilograms of microbes that live in stable equilibrium in these three regions perform their protective function using with us and our immune system. -

Histopathology of Barrett's Esophagus and Early-Stage
Review Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review Feng Yin, David Hernandez Gonzalo, Jinping Lai and Xiuli Liu * Department of Pathology, Immunology, and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL 32610, USA; fengyin@ufl.edu (F.Y.); hernand3@ufl.edu (D.H.G.); jinpinglai@ufl.edu (J.L.) * Correspondence: xiuliliu@ufl.edu; Tel.: +1-352-627-9257; Fax: +1-352-627-9142 Received: 24 October 2018; Accepted: 22 November 2018; Published: 27 November 2018 Abstract: Esophageal adenocarcinoma carries a very poor prognosis. For this reason, it is critical to have cost-effective surveillance and prevention strategies and early and accurate diagnosis, as well as evidence-based treatment guidelines. Barrett’s esophagus is the most important precursor lesion for esophageal adenocarcinoma, which follows a defined metaplasia–dysplasia–carcinoma sequence. Accurate recognition of dysplasia in Barrett’s esophagus is crucial due to its pivotal prognostic value. For early-stage esophageal adenocarcinoma, depth of submucosal invasion is a key prognostic factor. Our systematic review of all published data demonstrates a “rule of doubling” for the frequency of lymph node metastases: tumor invasion into each progressively deeper third of submucosal layer corresponds with a twofold increase in the risk of nodal metastases (9.9% in the superficial third of submucosa (sm1) group, 22.0% in the middle third of submucosa (sm2) group, and 40.7% in deep third of submucosa (sm3) group). Other important risk factors include lymphovascular invasion, tumor differentiation, and the recently reported tumor budding. In this review, we provide a concise update on the histopathological features, ancillary studies, molecular signatures, and surveillance/management guidelines along the natural history from Barrett’s esophagus to early stage invasive adenocarcinoma for practicing pathologists. -
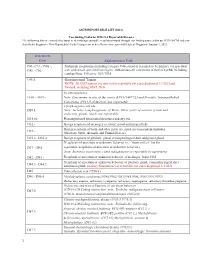
ASCR Reportable List ICD-10 Version
ASCR REPORTABLE LIST (2021) Casefinding Codes for ICD-O-3 Reportable Diseases The following lists are intended to assist in identifying reportable neoplasms found through casefinding sources that use ICD-10-CM codes to classify the diagnoses. New Reportable/ Code Changes are to be effective for cases with Date of Diagnosis January 1, 2021. ICD-10-CM Code Explanation of Code C00.- C43.-, C4A.-, Malignant neoplasms (excluding category C44), stated or resumed to be primary (of specified C45 - C96.- site) and certain specified histologies. Sebaceous cell carcinoma of skin of eyelid, including canthus Note: Effective 10/1/2018 C49.A- Gastrointestinal Tumors NOTE: All GIST tumors are now newly reportable for cases diagnosed 1/1/2021and forward, including GIST, NOS In-situ neoplasms D 00.- -D 09.- Note: Carcinoma in situ of the cervix (CIN 111-8077/2) and Prostatic lntraepithelial Carcinoma {PIN 111-8148/2) are not reportable Lymphangioma,any site D18.1 Note: Includes Lymphangiomas of Brain, Other parts of nervous system and endocrine glands, which are reportable D18.02 Hemangioma of intracranial structures and any site D32.- Benign neoplasm of meninges (cerebral, spinal and unspecified) Benign neoplasm of brain and other parts of central nervous system (includes D33.- Olfactory, Optic, Acoustic and Cranial Nerves) D35.2- D 35.4 Benign neoplasm of pituitary gland, craniopharyngeal duct and pineal gland Neoplasms of uncertain or unknown behavior (see "must collect" list for D37. - D41._ reportable neoplasms of uncertain or unknown behavior) Note: -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml.