(Ochnaceae) Leaf Extracts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Method to Estimate Dry-Kiln Schedules and Species Groupings: Tropical and Temperate Hardwoods
United States Department of Agriculture Method to Estimate Forest Service Forest Dry-Kiln Schedules Products Laboratory Research and Species Groupings Paper FPL–RP–548 Tropical and Temperate Hardwoods William T. Simpson Abstract Contents Dry-kiln schedules have been developed for many wood Page species. However, one problem is that many, especially tropical species, have no recommended schedule. Another Introduction................................................................1 problem in drying tropical species is the lack of a way to Estimation of Kiln Schedules.........................................1 group them when it is impractical to fill a kiln with a single Background .............................................................1 species. This report investigates the possibility of estimating kiln schedules and grouping species for drying using basic Related Research...................................................1 specific gravity as the primary variable for prediction and grouping. In this study, kiln schedules were estimated by Current Kiln Schedules ..........................................1 establishing least squares relationships between schedule Method of Schedule Estimation...................................2 parameters and basic specific gravity. These relationships were then applied to estimate schedules for 3,237 species Estimation of Initial Conditions ..............................2 from Africa, Asia and Oceana, and Latin America. Nine drying groups were established, based on intervals of specific Estimation -

Seeds and Plants Imported
' y Issued February 14,1923. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. INVENTORY OF SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM JANUARY 1 TO MARCH 31, 1920. (No. 62; Nos. 49124 TO 49796.) WASHINGTON: GOVERNMENT PRINTING OFFIC& Issued February 14,1923. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. INVENTORY OF SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM JANUARY 1 TO MARCH 31, 1920. (No. 62; Nos. 49124 TO 49796.) WASHINGTON: GOVERNMENT PRINTING OFFICE. 1923. CONTENTS. Tage. Introductory statement \ 1 Inventory . 5 Index of common and scientific names 87 ILLUSTRATIONS. Page. PLATE I. The fire-lily of Victoria Falls. (Buphane disticha (L. f.) Her- bert, S. P. I. No. 49256) 16 II. The m'bulu, an East African shrub allied to the mock orange. (Cardiogyne africana Bureau, S. P. I. No. 49319) 16 III. A latex-producing shrub from Mozambique. (Conopharyngia elegans Stapf, S. P. I. No. 49322) 24 IV. An East African relative of the mangosteen. (Garcinia living- stonei T. Anders., S. P. I. No. 49462) 24 V. A drought-resistant ornamental from Northern Rhodesia. (Ochna polyncura Gilg., S. P. I. No. 49595) 58 VI. A new relative of the Kafir orange. (Strychnos sp., S. P. I. No. 49599) 58 VII. Fruits of the maululu from the Zambezi Basin. (Canthium Ian- cifloruin Hiern, S. P. I. No. 49608) 58 VIII. A fruiting tree of the maululu. (Canthium landflorum Hiern, S. P. I. No. 49608) 58 in INVENTORY OF SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT IN- TRODUCTION DURING THE PERIOD FROM JAN- UARY 1 TO MARCH 31, 1920 (NO. -

Biomass Structure Relationships for Characteristic Species of the Western Kalahari, Botswana
UC Santa Barbara UC Santa Barbara Previously Published Works Title An analysis of structure: Biomass structure relationships for characteristic species of the western Kalahari, Botswana Permalink https://escholarship.org/uc/item/2mh197t1 Journal African Journal of Ecology, 52(1) ISSN 0141-6707 Authors Meyer, T D'Odorico, P Okin, GS et al. Publication Date 2014-03-01 DOI 10.1111/aje.12086 Peer reviewed eScholarship.org Powered by the California Digital Library University of California An analysis of structure: biomass structure relationships for characteristic species of the western Kalahari, Botswana Thoralf Meyer1,2*, Paolo D’Odorico1, Greg S. Okin3, Herman H. Shugart1, Kelly K. Caylor4, Frances C. O’Donnell4, Abi Bhattachan1 and Kebonyethata Dintwe3 1Department of Environmental Sciences, University of Virginia, Charlottesville, VA, 22904, U.S.A, 2Bureau of Economic Geology, University of Texas, Austin, TX, 78758, U.S.A, 3Department of Geography, University of California, Los Angeles, CA, 90095, U.S.A and 4Department of Civil and Environmental Engineering, Princeton University, Princeton, NJ, 08544, U.S.A Abstract Resume Savannah ecosystems are important carbon stocks on the Les ecosystemes de savane sont d’importants stocks de Earth, and their quantification is crucial for understand- carbone terrestres, et leur quantification est cruciale pour ing the global impact of climate and land-use changes in comprendre l’impact global des changements du climat et de savannahs. The estimation of aboveground/belowground l’utilisation des sols en savane. L’estimation de la biomasse plant biomass requires tested allometric relationships that vegetale au-dessus et en dessous de la surface exige des can be used to determine total plant biomass as a relations d’allometrie eprouvees qui puissent servir ad eter- function of easy-to-measure morphological indicators. -

Bulletin of the Natural History Museum
ISSN 0968-044 Bulletin of The Natural History Museum THE NATURAL HISTORY 22 KOV 2000 Q6NEKAI LIBRARY THE NATURAL HISTORY MUSEUM VOLUME 30 NUMBER 2 30 NOVEMBER 2000 The Bulletin of The Natural History Museum (formerly: Bulletin of the British Museum (Natural History) ), instituted in 1949, is issued in four scientific series, Botany, Entomology, Geology (incorporating Mineralogy) and Zoology. The Botany Series is edited in the Museum's Department of Botany Keeper of Botany: Dr R. Bateman Editor of Bulletin: Ms M.J. Short Papers in the Bulletin are primarily the results of research carried out on the unique and ever- growing collections of the Museum, both by the scientific staff and by specialists from elsewhere who make use of the Museum's resources. Many of the papers are works of reference that will remain indispensable for years to come. All papers submitted for publication are subjected to external peer review for acceptance. A volume contains about 160 pages, made up by two numbers, published in the Spring and Autumn. Subscriptions may be placed for one or more of the series on an annual basis. Individual numbers and back numbers can be purchased and a Bulletin catalogue, by series, is available. Orders and enquiries should be sent to: Intercept Ltd. P.O. Box 7 16 Andover Hampshire SP 10 1YG Telephone: (01 264) 334748 Fax: (01264) 334058 Email: [email protected] Internet: http://www.intercept.co.uk Claims for non-receipt of issues of the Bulletin will be met free of charge if received by the Publisher within 6 months for the UK, and 9 months for the rest of the world. -

SABONET Report No 18
ii Quick Guide This book is divided into two sections: the first part provides descriptions of some common trees and shrubs of Botswana, and the second is the complete checklist. The scientific names of the families, genera, and species are arranged alphabetically. Vernacular names are also arranged alphabetically, starting with Setswana and followed by English. Setswana names are separated by a semi-colon from English names. A glossary at the end of the book defines botanical terms used in the text. Species that are listed in the Red Data List for Botswana are indicated by an ® preceding the name. The letters N, SW, and SE indicate the distribution of the species within Botswana according to the Flora zambesiaca geographical regions. Flora zambesiaca regions used in the checklist. Administrative District FZ geographical region Central District SE & N Chobe District N Ghanzi District SW Kgalagadi District SW Kgatleng District SE Kweneng District SW & SE Ngamiland District N North East District N South East District SE Southern District SW & SE N CHOBE DISTRICT NGAMILAND DISTRICT ZIMBABWE NAMIBIA NORTH EAST DISTRICT CENTRAL DISTRICT GHANZI DISTRICT KWENENG DISTRICT KGATLENG KGALAGADI DISTRICT DISTRICT SOUTHERN SOUTH EAST DISTRICT DISTRICT SOUTH AFRICA 0 Kilometres 400 i ii Trees of Botswana: names and distribution Moffat P. Setshogo & Fanie Venter iii Recommended citation format SETSHOGO, M.P. & VENTER, F. 2003. Trees of Botswana: names and distribution. Southern African Botanical Diversity Network Report No. 18. Pretoria. Produced by University of Botswana Herbarium Private Bag UB00704 Gaborone Tel: (267) 355 2602 Fax: (267) 318 5097 E-mail: [email protected] Published by Southern African Botanical Diversity Network (SABONET), c/o National Botanical Institute, Private Bag X101, 0001 Pretoria and University of Botswana Herbarium, Private Bag UB00704, Gaborone. -

The Influence of Ants on the Insect Fauna of Broad
THE INFLUENCE OF ANTS ON THE INSECT FAUNA OF BROAD - LEAVED, SAVANNA TREES. by SUSAN GRANT Thesis submitted to Rhodes University in partial fulfilment for the degree of Master of Science Department of Zoology and Entomology Rhodes University Grahamstown SOUTH AFRICA May 1984 TO MY PARENTS AND SISTER, ROSEMARY ---- '.,. ... .... ...... ;; ............. .... :.:..:.: " \ ... .:-: ..........~.~ . .... ~ ... .... ......... • " ".! . " \ ......... :. ........ .................... FRONTISPIECE TOP RIGHT - A map of Southern Africa indicating the position of the Nylsvley Provincial Nature Reserve. TOP LEFT One of the dominant ant species, Crematogaster constructor, housed in a carton nest in a Terminalia sericea tree. R BOTTOM RIGHT - A sticky band of Formex which was applied to the trunk of trees to exclude ants. BOTTOM LEFT - Populations of the scale insect Ceroplastes rusci on the leaves of an unbanded Ochna pulchra shrub. CONTENTS Page ACKNOWLEDGEMENTS 1. ABSTRACT 2. INTRODUCTION 1 - 4 3. STUDY AREA 5 - 10 3.1 Abiotic components 3.2 Biotic components 4. GENERAL MATERIALS AND METHODS 11 - 14 4.1 Ant exclusion 4.2 Effect of banding 5. THE RESULTS OF BANDING 15 - 17 5.1 Effect on plant phenology 5.2 Effect on the insect fauna 6. THE ANT COMMUNITY 18 - 29 6.1 The ant species 6.2 Ant distribution and nesting sites 6.3 Field observations 7. SAMPLING OF BANDED AND UNBANDED TREES 30 - 44 7.1 Non-destructive sampling 7.2 Destructive pyrethrum sampling 8. ASSESSMENT OF HERBIVORY 45 - 55 8.1 Damage records on .banded and unbanded trees 9. DISCUSSION 56 - 60 APPENDICES 61 - 81 REFERENCES 82 - 90 ACKNOWLEDGEMENTS I would like to express my sincere thanks to Professor V.C. -

Taxonomy and Conservation Status of Pteridophyte Flora of Sri Lanka R.H.G
Taxonomy and Conservation Status of Pteridophyte Flora of Sri Lanka R.H.G. Ranil and D.K.N.G. Pushpakumara University of Peradeniya Introduction The recorded history of exploration of pteridophytes in Sri Lanka dates back to 1672-1675 when Poul Hermann had collected a few fern specimens which were first described by Linneus (1747) in Flora Zeylanica. The majority of Sri Lankan pteridophytes have been collected in the 19th century during the British period and some of them have been published as catalogues and checklists. However, only Beddome (1863-1883) and Sledge (1950-1954) had conducted systematic studies and contributed significantly to today’s knowledge on taxonomy and diversity of Sri Lankan pteridophytes (Beddome, 1883; Sledge, 1982). Thereafter, Manton (1953) and Manton and Sledge (1954) reported chromosome numbers and some taxonomic issues of selected Sri Lankan Pteridophytes. Recently, Shaffer-Fehre (2006) has edited the volume 15 of the revised handbook to the flora of Ceylon on pteridophyta (Fern and FernAllies). The local involvement of pteridological studies began with Abeywickrama (1956; 1964; 1978), Abeywickrama and Dassanayake (1956); and Abeywickrama and De Fonseka, (1975) with the preparations of checklists of pteridophytes and description of some fern families. Dassanayake (1964), Jayasekara (1996), Jayasekara et al., (1996), Dhanasekera (undated), Fenando (2002), Herat and Rathnayake (2004) and Ranil et al., (2004; 2005; 2006) have also contributed to the present knowledge on Pteridophytes in Sri Lanka. However, only recently, Ranil and co workers initiated a detailed study on biology, ecology and variation of tree ferns (Cyatheaceae) in Kanneliya and Sinharaja MAB reserves combining field and laboratory studies and also taxonomic studies on island-wide Sri Lankan fern flora. -

Survival, Regeneration and Leaf Biomass Changes in Woody Plants Following Spring Burns in Burkea Africana—Ochna Pulchra Savanna*
Bothalia 13, 3 & 4: 531-552 (1981) Survival, regeneration and leaf Biomass changes in woody plants following spring Burns in Burkea africana—Ochna pulchra Savanna* M. C. RUTHERFORD** ABSTRACT Effects of two intensities of spring Burn on various aspects of woody plants of a Burkea africana—Ochna pulchra Savanna after one growth season are given. Mortality of woody plants was very low with, for example, that of in dividuals of Ochna pulchra Being Between 1 and 5%. Some species where the aBove-ground parts were often Burned away completely, as in Grewia flavescens, no mortality of individuals occurred. Basal regeneration shoot mass was found to depend paraBolically on plant height while the ratio of leaf to twig mass in Basal shoot regeneration varied inversely with plant height in Ochna pulchra. The aBility of Ochna pulchra plants to produce new Basal shoots appeared to not only depend on size of the plant But also on the numBer of basal shoots present prior to the fire. In live Ochna pulchra plants Basal regeneration shoot Biomass per individual was found to increase exponentially with greater reduction in canopy leaf Biomass. This relation was also affected By possiBle direct heat effects. Basal shoot regeneration mass was found to vary greatly with species and varied from 0,7 g/individual for Dichapetalum cymosum to 285,6 g/individual for Euclea natalensis. There was a clear tendency for non-suffrutex shruB species to have greater mean Basal regeneration shoot mass per plant than that of most tree species. There was a compensatory effect in Ochna pulchra Between numBer and size of Basal regeneration shoots. -
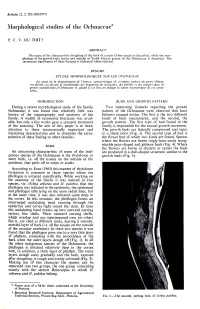
Morphological Studies of the Ochnaceae*
Bothalia 22, 2: 205-208(1977) Morphological studies of the Ochnaceae* P. C. V. DU TOIT f ABSTRACT The cause of the characteristic sloughing of the bark of certain Ochna species is described, while the mor phology of the growth buds, leaves and stipules of South African genera of the Ochnaceae is discussed. The taxonomic significance of these features is indicated where relevant. RESUME ETUDES MO RP HO LOG IQ UES SUR LES OCHNACEAE La cause de la desquamation de I'ecorce, caracteristique de certaines especes du genre Ochna, est deer it e\ on discute la morphologie des bourgeons de croissance, des feuilles et des stipules dans les genres sud-africains d'Ochnaceae et, quand il y a lieu, on indique la valeur taxonomique de ces carac- teres. INTRODUCTION BUDS AND GROWTH PATTERN During a recent morphological study of the family Two interesting features regarding the growth Ochnaceae, it was found that relatively little was pattern of the Ochnaceae were observed that have known of the organography and anatomy of the hitherto escaped notice. The first is the two different family. A wealth of taxonomic literature was avail kinds of buds encountered, and the second, the able, but only a few works gave a synoptic treatment growth pattern. The first type of bud found in the of the anatomy. The aim of this paper is to draw family is responsible for the annual growth increment. attention to these taxonomically important and The growth buds are laterally compressed and taper interesting characteristics and to stimulate the extra to a sharp point (Fig. -

Iso-Emissions.Pdf
Isoprene Emission Potential Monoterpene Emission Potential Family Species Common name C5 mg/gdw/h Key ref C10 mg/gdw/h Key ref Pinaceae Abies alba silver/European fir * <0.1 38,60 * 38 Pinaceae Abies balsamea balsam fir - [2] Pinaceae Abies bracteata bristlecone fir - [2] Pinaceae Abies cephalonica Greek fir * [2] Pinaceae Abies cilicica Turkish fir * [2] Pinaceae Abies concolor white fir - [2] Pinaceae Abies fraseri Fraser fir - [2] * [2] Pinaceae Abies insignis * [2] Pinaceae Abies l.var.arizonica corkbark fir - [2] Pinaceae Abies lasiocarpa subalpine fir - [2] Pinaceae Abies magnifica California red fir - [2] Pinaceae Abies marocana Morocco fir * [2] Pinaceae Abies nebrodensis Sicily fir - [2] Pinaceae Abies numidica Algerian fir * [2] Pinaceae Abies pardei * [2] Pinaceae Abies pinsapo Spanish fir * [2] Pinaceae Abies procera noble fir - [2] Pinaceae Abies spp. fir * <0.1 49 * 3±1.5 49,60 Leguminosae (Mimosaceae) Acacia baileyana cootamundra-wattle - [2] Leguminosae (Mimosaceae) Acacia berlandieri - 56 - 56 Leguminosae (Mimosaceae) Acacia dealbata - [40] * 0.85 [40] Leguminosae (Mimosaceae) Acacia decurrens green wattle - [2] Leguminosae (Mimosaceae) Acacia farnesiana sweet acacia - [3],56 - (*) 4.65 56;[3] Leguminosae (Mimosaceae) Acacia greggii cat's claw - [2],56 - 56 Leguminosae (Mimosaceae) Acacia longifolia (adult) - [40] * 2.04 [40] Leguminosae (Mimosaceae) Acacia longifolia (young) - [40] * 0.74 [40] Leguminosae (Mimosaceae) Acacia melanoxylon blackwood acacia - [2] Leguminosae (Mimosaceae) Acacia nigrescens African akazia * 110 106 * 0.7 106 Leguminosae (Mimosaceae) Acacia pennata - 107 Leguminosae (Mimosaceae) Acacia pentagona - 107 Leguminosae (Mimosaceae) Acacia podalyriaefolia pearl acacia - [2] Leguminosae (Mimosaceae) Acacia rigidula - 56 * 56 Leguminosae (Mimosaceae) Acacia sophorae Sydney wattle - [2] Leguminosae (Mimosaceae) Acacia spp. -

Geographical Distribution and Local Occurence of Dichapetalum
GEOGRAPHICALDISTRIBUTION AND LOCAL OCCURRENCE OF DToHAzETALUMCYMOSUM (HOOK) ENGL. (GTFBLAAR) rN NAMIBIAAND SURROUNDING AREAS R.I. DE SOUSA CORREIAAND L. YAN RENSBURG ResearchInstitute for ReclamationEcology, Potchefstroom University for ChristianHigher Education P.O. Box 19752,Noordbrug,2522,RSA ABSTRACT INTRODUCTION This paper discussesDichapetalum cymosum's habitat, The family Dichapetaluminclude two genera: Dichapetalum coveringits distributionacross southern Angola, Namibia, and Tapura.Dichapetalum is representedby about200 spp., Botswanaand South Africa in orderto ascertainit's ecological trees,shrubs and rhizomatousshrublets: cosmopolitan in nicheas delimitedby someclimatological and edaphic factors. tropicalcoutries, rare in sub tropics.There are threespecies From this study the distributionarea of D. cymosumwould o'fDichapetalum in southernAfrica (Dyke 1975). seem to extendfrom the north-westernlimits of the loose Kalaharisands, in southernAngola (Cunene River basin - Dichapetalumcymosum, is a well-knownpoisonous mediumcourse), across north-easternNamibia (Okavango rhizomatousplant endemic to SouthernAfrica, and knownto River basin and the endorreicdry system of various occurin cedain areas of the dry savanna, mostly in the Kalahari omiramba),across Botswana(southwest of the Okavango Basin(Dreyer 1975, Smith 1966). lts occurrencehas also Deltaand of the MakgadikgadiPan and northof the Molopo beenreported in Angola(south), Zambia (south), Zimbabwe andacross the Limpopo River basins) into South Africa, across (west),Botswana (central), Namibia (north-east) and South the Marico/Crocodile,Limpopo, Nyl, Pienaarsand Elands/ Africa(north-west) (Vahrmeijer, 1981). D. cymosumcan be Olifantriver basins. typifiedas a dwarfshrublet with deep-seated,rhyzomatous roots.The leavesare allternately arranged and are generally The generalecological characteristics of the distributionarea soft and hairywhile the inflorescence,an axillarycyme, of D. cymosumseems to be a sandy,well drained and nutrient produceswhite flowers that developsinto drupaceous fruits. -

Tree Types of the World Map
Abarema abbottii-Abarema acreana-Abarema adenophora-Abarema alexandri-Abarema asplenifolia-Abarema auriculata-Abarema barbouriana-Abarema barnebyana-Abarema brachystachya-Abarema callejasii-Abarema campestris-Abarema centiflora-Abarema cochleata-Abarema cochliocarpos-Abarema commutata-Abarema curvicarpa-Abarema ferruginea-Abarema filamentosa-Abarema floribunda-Abarema gallorum-Abarema ganymedea-Abarema glauca-Abarema idiopoda-Abarema josephi-Abarema jupunba-Abarema killipii-Abarema laeta-Abarema langsdorffii-Abarema lehmannii-Abarema leucophylla-Abarema levelii-Abarema limae-Abarema longipedunculata-Abarema macradenia-Abarema maestrensis-Abarema mataybifolia-Abarema microcalyx-Abarema nipensis-Abarema obovalis-Abarema obovata-Abarema oppositifolia-Abarema oxyphyllidia-Abarema piresii-Abarema racemiflora-Abarema turbinata-Abarema villifera-Abarema villosa-Abarema zolleriana-Abatia mexicana-Abatia parviflora-Abatia rugosa-Abatia spicata-Abelia corymbosa-Abeliophyllum distichum-Abies alba-Abies amabilis-Abies balsamea-Abies beshanzuensis-Abies bracteata-Abies cephalonica-Abies chensiensis-Abies cilicica-Abies concolor-Abies delavayi-Abies densa-Abies durangensis-Abies fabri-Abies fanjingshanensis-Abies fargesii-Abies firma-Abies forrestii-Abies fraseri-Abies grandis-Abies guatemalensis-Abies hickelii-Abies hidalgensis-Abies holophylla-Abies homolepis-Abies jaliscana-Abies kawakamii-Abies koreana-Abies lasiocarpa-Abies magnifica-Abies mariesii-Abies nebrodensis-Abies nephrolepis-Abies nordmanniana-Abies numidica-Abies pindrow-Abies pinsapo-Abies