Tree Types of the World Map
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cunninghamia : a Journal of Plant Ecology for Eastern Australia
27 Flora conservation issues at Kinchega National Park, western NSW Tony D. Auld and Andrew J. Denham Auld, Tony D., and Denham, Andrew J. (Biodiversity Research and Management Division, NSW National Parks & Wildlife Service, PO Box 1967 Hurstville NSW 2220 email: [email protected]) 2001. Flora conservation issues at Kinchega National Park, western NSW. Cunninghamia 7(1): 27–41. Kinchega National Park reserves significant stands of Eucalyptus largiflorens open woodland on the Darling River floodplain, low open Maireana pyramidata shrubland and Casuarina pauper/Alectryon oleifolius open woodland on dune systems. We identify four key issues for the conservation of flora in Kinchega National Park, western NSW. These are: 1) There is an urgent need to initiate regeneration in a number of long-lived perennial trees and shrubs. Failure to do so will lead to local population declines and extinction in a number of species. Reduction in grazing impacts of rabbits and goats is needed. Some degree of rabbit control has been achieved over the last few years through a combination of the effects of the rabbit calicivirus disease (RCD) and an extensive rabbit control program for the reserve. 2) The need to initiate a water plan of management for the reserve to overcome the problem of changes in water flows, flood periodicity and flood magnitude that have occurred in response to water regulation activities on the Darling River. 3) Management of several threatened species and ecological communities on the reserve, in particular the nationally vulnerable species Acacia carneorum and Solanum karsense. Kinchega NP is the only conservation reserve containing populations of these species and these populations are significant for both species. -

Australian Native Plants Society Canberra Region(Inc)
AUSTRALIAN NATIVE PLANTS SOCIETY CANBERRA REGION (INC) Journal Vol. 17 No. 4 December 2012 ISSN 1447-1507 Print Post Approved PP299436/00143 Contents ANPS Canberra Region Report 1 Whose Bean genus is that? 3 Winter Walks 6 Signs renewal for Frost Hollow to Forest Walk 16 Touga Road Touring 21 Study Group Snippets 25 Acacia Study Group Field Trip 27 ANPSA Study Groups 34 ANPS contacts and membership details inside back cover Cover: Correa reflexa, Kambah Pool, North; Photo: Martin Butterfield Journal articles The deadline dates for submissions are 1 February The Journal is a forum for the exchange of members' (March), 1 May (June), 1 August (September) and and others' views and experiences of gardening with, 1 November (December). Send articles or photos to: propagating and conserving Australian plants. Journal Editor All contributions, however short, are welcome. Gail Ritchie Knight Contributions may be typed or handwritten, and 1612 Sutton Road accompanied by photographs and drawings. Sutton NSW 2620 e-mail: [email protected] Submit photographs as either electronic files, tel: 0416 097 500 such as JPGs, or prints. Please enclose a stamped, self-addressed envelope if you would like your prints Paid advertising is available in this Journal. Details returned. If possible set your digital camera to take from the Editor. high resolution photos. If photos cannot be emailed, Society website: http://nativeplants-canberra.asn.au make a CD and send it by post. If you have any Printed by Elect Printing, Fyshwick, ACT queries please contact the editor http://www.electprinting.com.au/ Original text may be reprinted, unless otherwise indicated, provided an acknowledgement for the source is given. -

Creating Jobs, Protecting Forests?
Creating Jobs, Protecting Forests? An Analysis of the State of the Nation’s Regional Forest Agreements Creating Jobs, Protecting Forests? An Analysis of the State of the Nation’s Regional Forest Agreements The Wilderness Society. 2020, Creating Jobs, Protecting Forests? The State of the Nation’s RFAs, The Wilderness Society, Melbourne, Australia Table of contents 4 Executive summary Printed on 100% recycled post-consumer waste paper 5 Key findings 6 Recommendations Copyright The Wilderness Society Ltd 7 List of abbreviations All material presented in this publication is protected by copyright. 8 Introduction First published September 2020. 9 1. Background and legal status 12 2. Success of the RFAs in achieving key outcomes Contact: [email protected] | 1800 030 641 | www.wilderness.org.au 12 2.1 Comprehensive, Adequate, Representative Reserve system 13 2.1.1 Design of the CAR Reserve System Cover image: Yarra Ranges, Victoria | mitchgreenphotos.com 14 2.1.2 Implementation of the CAR Reserve System 15 2.1.3 Management of the CAR Reserve System 16 2.2 Ecologically Sustainable Forest Management 16 2.2.1 Maintaining biodiversity 20 2.2.2 Contributing factors to biodiversity decline 21 2.3 Security for industry 22 2.3.1 Volume of logs harvested 25 2.3.2 Employment 25 2.3.3 Growth in the plantation sector of Australia’s wood products industry 27 2.3.4 Factors contributing to industry decline 28 2.4 Regard to relevant research and projects 28 2.5 Reviews 32 3. Ability of the RFAs to meet intended outcomes into the future 32 3.1 Climate change 32 3.1.1 The role of forests in climate change mitigation 32 3.1.2 Climate change impacts on conservation and native forestry 33 3.2 Biodiversity loss/resource decline 33 3.2.1 Altered fire regimes 34 3.2.2 Disease 35 3.2.3 Pest species 35 3.3 Competing forest uses and values 35 3.3.1 Water 35 3.3.2 Carbon credits 36 3.4 Changing industries, markets and societies 36 3.5 International and national agreements 37 3.6 Legal concerns 37 3.7 Findings 38 4. -

Examining the Acacia Boormanii Complex (Fabaceae: Mimosoideae); Recognition of a New Subspecies
Muelleria 37: 23–32 Published online in advance of the print edition, 28 June 2018 Examining the Acacia boormanii complex (Fabaceae: Mimosoideae); recognition of a new subspecies Kelsey J. Tucker1, Daniel J. Murphy2, Neville Walsh2,3 1 Department of Environment, Land, Water and Planning, 1–7 Taylor St, Epsom, Victoria 3551 2 Royal Botanic Gardens Victoria, Melbourne, Victoria 3004 3 Corresponding author: [email protected] Introduction Abstract The iconic genus Acacia Mill. (Leguminosae: Mimosoideae) is the largest A morphometric analysis of specimens angiosperm genus in Australia, consisting of over 1000 species (Miller determined as Acacia boormanii Maiden and A. infecunda Molyneux et al. 2011, Maslin 2015). Acacia boormanii Maiden (syn. A. hunteriana & Forrester supported a distinctive N.A.Wakef.) was described as a species of scattered and restricted population centred on Mt Typo occurrence in south-eastern Australia (Maiden 1916). As currently in north-eastern Victoria, which understood, its natural range extends from south of Thredbo Village, is described here as A. boormanii New South Wales (NSW), to near Buchan, Victoria, mostly south of the subsp. gibba K.J.Tucker subsp. nov. The characters that best separate Great Dividing Range, with isolated occurrences near Cooma, NSW, the new subspecies are the phyllode and Myrtleford, Victoria (Maslin 2001). It is common in cultivation and width, the indentation of the phyllode has become naturalised in a few areas outside its natural range (e.g. margins at the gland, and the shape http://avh.ala.org.au/occurrences/search?taxa=acacia+boormanii#tab_ of the phyllode apex. Neither Acacia mapView). -

Identifying Climate Refugia for Key Species in New South Wales - Final Report from the Bionode of the NSW Adaptation Hub
Identifying Climate Refugia for Key Species in New South Wales - Final Report from the BioNode of the NSW Adaptation Hub Linda J. Beaumont, John B. Baumgartner, Manuel Esperón-Rodríguez, David Nipperess 1 | P a g e Report prepared for the NSW Office of Environment and Heritage as part of a project funded by the NSW Adaptation Research Hub–Biodiversity Node. While every effort has been made to ensure all information within this document has been developed using rigorous scientific practice, readers should obtain independent advice before making any decision based on this information. Cite this publication as: Beaumont, L. J., Baumgartner, J. B., Esperón-Rodríguez, M, & Nipperess, D. (2019). Identifying climate refugia for key species in New South Wales - Final report from the BioNode of the NSW Adaptation Hub, Macquarie University, Sydney, Australia. For further correspondence contact: [email protected] 2 | P a g e Contents Acknowledgements ................................................................................................................................. 5 Abbreviations .......................................................................................................................................... 6 Glossary ................................................................................................................................................... 7 Executive summary ................................................................................................................................. 8 Highlights -
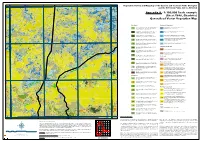
Appendix 9 - 1:100,000 Scale Example (Sheet 5648, Charlotte) Generalised Vector Vegetation Map
133°30'E 133°40'E 133°50'E 134°E 24°30'S Vegetation Survey and Mapping of the Eastern and Southern Finke Bioregion 24°30'S and the NT Stony Plains Inliers, NT & SA Appendix 9 - 1:100,000 Scale example (Sheet 5648, Charlotte) Generalised Vector Vegetation Map Woodland Chenopod Shrubland Acacia aneura ( Mulga) Low Open Woodland TO Tall Open Shrubland of Atriplex nummularia (Old man saltbush) Low Sparse Chenopod 1 Acacia estrophiolata (Ironwood) on clay loam plains and red earth 4 shrubland over Low Sparse Tussock grasses. soils+/- Atriplex vesicaria and Eragrostis eriopoda . Acacia georginae / Acacia cambagei ( Gidgee) Low Woodland to Tall Atriplex vesicaria (Pop saltbush) Low Open Chenopod Shrubland.+/- 2 Shrubland.+/- Eucalyptus coolabah subsp. Arida , Codonocarpus 5 Maireana astrotricha over tussock grasses. cotinifolius , Eulalia aurea, Eriachne ovata and Atriplex vesicaria . Eucalyptus coolabah subsp. arida (Coolabah) Woodland. +/- Maireana aphylla (Cottonbush) Low Sparse Chenopod Shrubland. +/- 12 Muehlenbeckia florulenta , Acacia aneura , Senna artemisioides ssp. 8 Fimbristylis dichotoma , Dactyloctenium radulans and Eragrostis dielsii. Filifolia , Marsilea sp ., Cynodon dactylon , and Cenchrus ciliaris . Maireana astrotricha (Low bluebush) Low Sparse Chenopod Shrubland Eucalyptus camaldulensis var. obtusa (River red gum) Woodland.+/- TO Sparse shrubland of Senna artemisioides n. coriacea and 13 Eucalyptus coolabah subsp. arida , Cynodon dactylon , Eulalia aurea and 9 Eremophila duttonii (Harlequin fuchsia bush). Cyperus gymnocaulos . 24°40'S Hakea leucoptera subsp. leucoptera (Needlewood) Open Woodland. +- Sclerolaena (mixed) Low Sparse Chenopod Shrubland.+/- Enneapogon 24°40'S 14 Eremophila sturtii , Senna artemisioides ssp. filifolia , Hakea leucoptera 15 avenaceus Aristida contorta , Sporobolus actinocladus . subsp. leucoptera and Triodia basedowii . Acacia calcicola (Northern Myall) Sparse Woodland +/- Eremophila Samphire Shrubland 23 duttonii , Acacia calcicola , Atriplex vesicaria , Maireana georgei and mixed short grasses. -

Improvement of Seed Germination in Three Important Conifer Species by Gibberellic Acid (GA3)
Volume 11(2) Improvement of seed germination in three important conifer species by Gibberellic acid (GA3). Improvement of seed germination in three important conifer species by Gibberellic acid (GA3). B. S. Rawat1, C. M. Sharma2 and S. K. Ghildiyal3 Department of Forestry, Post Box # 76, HNB Garhwal University, Srinagar Garhwal-246 174 (Uttaranchal) 1. [email protected] 2. [email protected] [email protected] December 2006 Download at: http://www.lyonia.org/downloadPDF.php?pdfID=283.486.1 Improvement of seed germination in three important conifer species by Gibberellic acid (GA3). Abstract Results pertaining to the germination percentage of pre-soaked seeds in a series of temperature regimes viz., 100C, 150C, 200C and 250C have revealed significant increase among seed sources in each of the three conifer species of Garhwal Himalaya. Soaking of the seeds for 24 hours in GA3 solution had shown maximum germination in A. pindrow (45.0±4.19%), C. torulosa (57.0±3.40%) and P. smithiana (56±6.01%) as compared to untreated (control) seeds. It has also been observed that GA3 treatment caused an appreciable shortening of the germination period by 10 days. Therefore, seeds of these commercially important tree species should be pre-treated particularly with GA3 for 24 hours for getting enhanced germination. It is important to point out here that the seeds of each of the three species reflect poor germination in nature due to snow cover, seed decay, prevalence of excess water and lack of maintenance, however, because of increasing demand for large quantities of tree seeds for reforestation programmes, pre-sowing treatments are useful to improve the rate and percentage of germination. -

Variation in Soil CO2 Efflux in Pinus Wallichiana and Abies Pindrow
rch: O ea pe es n A R t c s c e e Sundarapandian and Dar, Forest Res 2013, 3:1 r s o s Forest Research F DOI: 10.4172/2168-9776.1000116 Open Access ISSN: 2168-9776 Research Article Open Access Variation in Soil CO2 Efflux in Pinus Wallichiana and Abies Pindrow Temperate Forests of Western Himalayas, India SM Sundarapandian* and Javid Ahmad Dar Department of Ecology and Environmental Sciences, School of life Sciences, Pondicherry University, Puducherry, India Abstract Soil CO2 efflux was measured by alkali absorption method from April to December 2012 in two different forest types, i.e., Pinus wallichiana and Abies pindrow, with three replicate plots in each forest type. Soil CO2 efflux was found maximum in July and minimum in December in both the forest types. Significantly (P<0.001) greater soil CO2 efflux was measured inPinus wallichiana forest compared to Abies pindrow forest throughout the study period. The -2 -1 range of soil CO2 efflux (mg CO2 m hr ) from the soil was 126-427 in Abies pindrow forest and 182-646 in Pinus wallichiana forest. Soil CO2 efflux showed greater values in Pinus wallichiana forest than Abies pindrow forest, which could be attributed to greater tree density, tree biomass, shrub density, shrub biomass, forest floor litter and moisture. Soil CO2 efflux also showed significant positive relationship with air temperature. In addition to that the altitudinal difference may be one of the reasons for variation in soil CO2 efflux between the two forest types. This result also indicates that at higher altitude even a small difference in elevation (100 m) alter the functional attributes of the ecosystem. -

ACT, Australian Capital Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

MVG 16 Acacia Shrublands DRAFT
MVG 16 - ACACIA SHRUBLANDS Acacia hillii, Tanami Desert, NT (Photo: D. Keith) Overview The overstorey of MVG 16 is dominated by multi-stemmed acacia shrubs. The most widespread species is Acacia aneura (mulga). Mulga vegetation takes on a variety of structural expressions and is consequently classified partly within MVG 16 where the overstorey is dominated by multi-stemmed shrubs, partly within MVG 6 in accordance with the Kyoto Protocol definition of forest cover in Australia (trees > 2 m tall and crown cover > 20%, foliage projective cover > 10%); and partly within MVG 13 where the woody dominants are predominantly single-stemmed, but with crown cover less than 20%. Occurs where annual rainfall is below 250mm in southern Australia and below 350mm in northern Australia (Hodgkinson 2002; Foulkes et al. 2014). Species composition varies along rainfall gradients, with substrate and rainfall seasonality (Beadle 1981; Johnson and Burrows 1994). Transitions into MVG 13 Acacia woodlands with higher rainfall and varying soil types. Is most commonly found on red earth soils (Hodgkinson 2002). Facts and figures Major Vegetation Group MVG 16 - Acacia Shrublands Major Vegetation Subgroups 20. Stony mulga woodlands and shrublands NSW, (number of NVIS descriptions) NT, QLD, SA, WA 23. Sandplain Acacia woodlands and shrublands NSW, NT, QLD, SA, WA Typical NVIS structural formations Shrubland (tall, mid,) Open shrubland (tall, mid,) Sparse shrubland (tall, mid,) Number of IBRA regions 53 Most extensive in IBRA region Est. pre-1750 and present : Great Victoria Desert (WA and SA) Estimated pre-1750 extent (km2) 865 845 Present extent (km2) 851 274 Area protected (km2) 85 444 Acacia ligulata (sandhill wattle), SA (Photo: M. -

Legumes of Wallace Desert Gardens
Bulletin of The Desert Legume Program of The Boyce Thompson Southwestern Arboretum and The University of Arizona Volume 18, Number 2 August 2006 Legumes of Wallace Desert Gardens Pamela Slate standing relationship between our Desert Gardens reviews and Botanic Coordinator organizations, one I see growing ever approves appropriate on-site Wallace Desert Gardens stronger year after year.” projects of mutual benefit. Wallace Desert Gardens is a Matthew B. Johnson non-profit foundation [(502(c)(3) In the mid-1980’s, the Program Manager and Curator under IRS rules] that was created in Wallace’s moved, complete with their Desert Legume Program 1993, well after much of the garden plant collection, from a Paradise was established. Its mission was Valley acre to a Scottsdale The virtues of desert legumes written by HB, as he was fondly subdivision where they purchased captured the attention of H.B. and known, to reflect the original intent of numerous acre-plus lots. At the time, Jocelyn M. Wallace when they first the foundation: HB had “no idea it would be bigger learned of the Desert Legume than a two-acre garden.” Although Program (DELEP) in 1989, about a Wallace Desert Gardens is a he “knew nothing of desert plants year after the program was founded collection of the world’s deserts when he moved to Arizona” in the at the University of Arizona. They plants located at an elevation of early 1980’s, they quickly became his understood the importance of some 2400 feet. Founded by passion. Today the garden legumes’ potential applications H.B. -

Impacts of Land Clearing
Impacts of Land Clearing on Australian Wildlife in Queensland January 2003 WWF Australia Report Authors: Dr Hal Cogger, Professor Hugh Ford, Dr Christopher Johnson, James Holman & Don Butler. Impacts of Land Clearing on Australian Wildlife in Queensland ABOUT THE AUTHORS Dr Hal Cogger Australasian region” by the Royal Australasian Ornithologists Union. He is a WWF Australia Trustee Dr Hal Cogger is a leading Australian herpetologist and former member of WWF’s Scientific Advisory and author of the definitive Reptiles and Amphibians Panel. of Australia. He is a former Deputy Director of the Australian Museum. He has participated on a range of policy and scientific committees, including the Dr Christopher Johnson Commonwealth Biological Diversity Advisory Committee, Chair of the Australian Biological Dr Chris Johnson is an authority on the ecology and Resources Study, and Chair of the Australasian conservation of Australian marsupials. He has done Reptile & Amphibian Specialist Group (IUCN’s extensive research on herbivorous marsupials of Species Survival Commission). He also held a forests and woodlands, including landmark studies of Conjoint Professorship in the Faculty of Science & the behavioural ecology of kangaroos and wombats, Mathematics at the University of Newcastle (1997- the ecology of rat-kangaroos, and the sociobiology of 2001). He is a member of the International possums. He has also worked on large-scale patterns Commission on Zoological Nomenclature and is a in the distribution and abundance of marsupial past Secretary of the Division of Zoology of the species and the biology of extinction. He is a member International Union of Biological Sciences. He is of the Marsupial and Monotreme Specialist Group of currently the John Evans Memorial Fellow at the the IUCN Species Survival Commission, and has Australian Museum.