Geographic Patterns in Morphometric and Genetic Variation for Coyote Populations with Emphasis on Southeastern Coyotes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Yukon College Student Survey Results
Yukon College Student Survey Results 2014-15 Dawn Macdonald Institutional Research and Planning Officer December 24, 2014 Yukon College Student Survey Results: 2014-15 TABLE OF CONTENTS HIGHLIGHTS ...................................................................................................................................................... 3 PURPOSE AND METHODS ............................................................................................................................. 5 RESULTS .............................................................................................................................................................. 6 STUDENT SATISFACTION ............................................................................................................................................ 6 More than nine out of ten would recommend their program or courses .................................... 6 Nearly everyone is satisfied with the quality of instruction ............................................................. 7 More than nine out of ten are satisfied with course content ........................................................... 7 Nine out of ten are satisfied with support services ............................................................................. 8 Nine out of ten are satisfied with program advising and library resources ............................... 9 Satisfaction with spaces for group work lags behind satisfaction with social spaces and study spaces ................................................................................................................................................... -

The Alaska-Yukon Region of the Circumboreal Vegetation Map (CBVM)
CAFF Strategy Series Report September 2015 The Alaska-Yukon Region of the Circumboreal Vegetation Map (CBVM) ARCTIC COUNCIL Acknowledgements CAFF Designated Agencies: • Norwegian Environment Agency, Trondheim, Norway • Environment Canada, Ottawa, Canada • Faroese Museum of Natural History, Tórshavn, Faroe Islands (Kingdom of Denmark) • Finnish Ministry of the Environment, Helsinki, Finland • Icelandic Institute of Natural History, Reykjavik, Iceland • Ministry of Foreign Affairs, Greenland • Russian Federation Ministry of Natural Resources, Moscow, Russia • Swedish Environmental Protection Agency, Stockholm, Sweden • United States Department of the Interior, Fish and Wildlife Service, Anchorage, Alaska CAFF Permanent Participant Organizations: • Aleut International Association (AIA) • Arctic Athabaskan Council (AAC) • Gwich’in Council International (GCI) • Inuit Circumpolar Council (ICC) • Russian Indigenous Peoples of the North (RAIPON) • Saami Council This publication should be cited as: Jorgensen, T. and D. Meidinger. 2015. The Alaska Yukon Region of the Circumboreal Vegetation map (CBVM). CAFF Strategies Series Report. Conservation of Arctic Flora and Fauna, Akureyri, Iceland. ISBN: 978- 9935-431-48-6 Cover photo: Photo: George Spade/Shutterstock.com Back cover: Photo: Doug Lemke/Shutterstock.com Design and layout: Courtney Price For more information please contact: CAFF International Secretariat Borgir, Nordurslod 600 Akureyri, Iceland Phone: +354 462-3350 Fax: +354 462-3390 Email: [email protected] Internet: www.caff.is CAFF Designated -

Eastern Coyote Assessment - 1999
EASTERN COYOTE ASSESSMENT - 1999 By Walter J. Jakubas Mammal Group Leader Maine Department of Inland Fisheries and Wildlife 650 State Street Bangor, Maine 04401 June 1999 EASTERN COYOTE ASSESSMENT TABLE OF CONTENTS Page INTRODUCTION...........................................................................................................4 NATURAL HISTORY.....................................................................................................5 Distribution and Taxonomy.................................................................................5 Food Habits ........................................................................................................6 Interactions with Other Species..........................................................................7 Reproduction ....................................................................................................10 Dispersal ..........................................................................................................11 Social Behavior.................................................................................................12 Mortality............................................................................................................13 MANAGEMENT ..........................................................................................................16 Regulatory Authority .........................................................................................16 Past Goals and Objectives ...............................................................................18 -

Taiga Plains
ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES Taiga Plains Ecosystem Classification Group Department of Environment and Natural Resources Government of the Northwest Territories Revised 2009 ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES TAIGA PLAINS This report may be cited as: Ecosystem Classification Group. 2007 (rev. 2009). Ecological Regions of the Northwest Territories – Taiga Plains. Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, NT, Canada. viii + 173 pp. + folded insert map. ISBN 0-7708-0161-7 Web Site: http://www.enr.gov.nt.ca/index.html For more information contact: Department of Environment and Natural Resources P.O. Box 1320 Yellowknife, NT X1A 2L9 Phone: (867) 920-8064 Fax: (867) 873-0293 About the cover: The small photographs in the inset boxes are enlarged with captions on pages 22 (Taiga Plains High Subarctic (HS) Ecoregion), 52 (Taiga Plains Low Subarctic (LS) Ecoregion), 82 (Taiga Plains High Boreal (HB) Ecoregion), and 96 (Taiga Plains Mid-Boreal (MB) Ecoregion). Aerial photographs: Dave Downing (Timberline Natural Resource Group). Ground photographs and photograph of cloudberry: Bob Decker (Government of the Northwest Territories). Other plant photographs: Christian Bucher. Members of the Ecosystem Classification Group Dave Downing Ecologist, Timberline Natural Resource Group, Edmonton, Alberta. Bob Decker Forest Ecologist, Forest Management Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Hay River, Northwest Territories. Bas Oosenbrug Habitat Conservation Biologist, Wildlife Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, Northwest Territories. Charles Tarnocai Research Scientist, Agriculture and Agri-Food Canada, Ottawa, Ontario. Tom Chowns Environmental Consultant, Powassan, Ontario. Chris Hampel Geographic Information System Specialist/Resource Analyst, Timberline Natural Resource Group, Edmonton, Alberta. -

Volume 7: the Boreal Forest TEACHING
TEACHING KIT Volume 7: The Boreal Forest National Forest Week 2006: September 24 to 30 The Canadian Forestry Association is pleased to announce that after careful consultation and consideration, in 2006 National Forest Week will move from spring to fall. Based on a 100-year legacy of facilitating forest education, the CFA believes this new approach will spur increased year-round learning opportunities for interested Canadians. Trends show that early education is key to capturing and fostering the interest of youth towards volunteerism, higher education and careers in the forest and environmental sectors. Developing the forestry leaders of tomorrow is critical to ensuring sustainability of our natural resources and the socio-economic and health benefits they provide for all Canadians. Integral to this learning process is the Canada's Forests Teaching Kit series, which has become the cornerstone of CFA’s education and outreach initiatives. Available free to educators, these kits provide tools for helping youth better understand the value of forest resources and the importance of using them wisely. Beginning in 2006, the annual kit publication date will change to coin- cide with National Forest Week each September. This is in response to overwhelming feedback from teachers across Canada indicating a strong preference to receive these materials at commencement of the school year for increased and enhanced integration into teaching plans and other outreach activities. In keeping with tradition, I invite you to join the CFA in celebrating Canada’s forests — in September and year-round: plant a tree, walk through a forest or learn about forest management. Your local forestry association can provide more ideas, teaching materials and information about forest activities in your area. -

Regional Ecosystems of West-Central Yukon
Regional Ecosystems of West-Central Yukon PART 1: ECOSYSTEM DESCRIPTIONS March 2012 Prepared for Environment Yukon Prepared by Makonis Consulting Ltd. In association with S. Francis Consulting Inc. Site Planning + GIS John Meikle ISBN 978-1-55362-635-0 Copies of this report, including a digital version, are available at: c/o Ecological and Landscape Classification (ELC) Coordinator Environmental Planning Policy and Planning Branch Environment Yukon Box 2703, Whitehorse, Yukon Y1A 2C6 ph. (867) 667-3081 fax: (867) 393-6213 Visit the Yukon Ecological and Landscape Classification Program Website at www.env.gov.yk.ca/elc SUGGESTED CITATIONS Report Citation: “Grods, J., S.R. Francis, J.C. Meikle and S. Lapointe. 2012. Regional Ecosystems of West-Central Yukon, Part 1: Ecosystem descriptions. Report prepared for Environment, Government of Yukon by Makonis Consulting Ltd. and Associates, West Kelowna, BC.” Spatial Data Citation: “Grods, J., S.R. Francis, J.C. Meikle and S. Lapointe. 2012. West-central broad ecosystems (Version 1.1). Downloaded from Yukon government ftp site on XXX (replace XXX with date of download). Spatial data created for Environment, Government of Yukon by Makonis Consulting Ltd. and Associates, West Kelowna, BC.” DOCUMENT PREPARATION: The Ecosystems of West-central Yukon was produced by Makonis Consulting Ltd. and associates for Environment, Government of Yukon (the data Custodian). The project was managed by Nadele Flynn, Coordinator, Ecological and Landscape Classification (ELC) Program, Environmental Planning Branch, Government of Yukon. Although every effort has been made to ensure the correctness of the data in the Ecosystems of West-central Yukon report and spatial products, there still may be errors. -

Yukon River Basin Study Project Report
YUKON RIVER BASIN STUDY PROJECT REPORT: WILDLIFE NO. 1 FURBEARER INVENTORY, HABITAT ASSESSMENT AND TRAPPER UTILIZATION OF THE YUKON RIVER BASIN B.G. Slough and R.H. Jessup Wildlife Management Branch Yukon Department of Renewable Resources Box 2703 Whitehorse, Yukon Y1A 2C6 January 1984 i This report was funded by the Yukon River Basin Committee (jointly with Yukon Department of Renewable Resources) under the terms of "An Agreement Respecting Studies and Planning of Water Resources in the Yukon River Basin" between Canada, British Columbia and Yukon. The views, conclusions and recommendations are those of the authors and not necessarily those of the Yukon River Basin Committee or the Government of Canada, British Columbia and Yukon. ii ABSTRACT The Yukon Department of Renewable Resources conducted furbearer inventory studies in the Canadian Yukon River Basin in 1982 and 1983. Field studies included beaver (Castor canadensis) food cache and colony site surveys, winter track-count sampling and muskrat (Ondatra zibethica) pushup surveys. Data from the surveys were analyzed in conjunction with ongoing trapper questionnaire and historical fur harvest data sources to characterize furbearer population distributions, levels, trends and habitats. Historical and present fur harvest and trapping activity are described. The fur resource capability and problems and issues associated with impacts on furbearer populations, habitats and user groups are discussed. The populations of wolves Canis lupus, red fox Vulpes fulva, coyote .Q..:.. latrans, red squirre 1 Tamiasciurus hudsonicus, wease 1 Mustela erminea, marten Martes americana, mink Mustela vison, otter Lutra canadensis, wolverine Gulo luscus, beaver, muskrat and lynx Lynx canadensis are all widely distributed within the Yukon River Basin. -

Yakutat Tlingit and Wrangell-St. Elias National Park and Preserve: an Ethnographic Overview and Assessment
Portland State University PDXScholar Anthropology Faculty Publications and Presentations Anthropology 2015 Yakutat Tlingit and Wrangell-St. Elias National Park and Preserve: An Ethnographic Overview and Assessment Douglas Deur Portland State University, [email protected] Thomas Thornton University of Oxford Rachel Lahoff Portland State University Jamie Hebert Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/anth_fac Part of the Social and Cultural Anthropology Commons Let us know how access to this document benefits ou.y Citation Details Deur, Douglas; Thornton, Thomas; Lahoff, Rachel; and Hebert, Jamie, "Yakutat Tlingit and Wrangell-St. Elias National Park and Preserve: An Ethnographic Overview and Assessment" (2015). Anthropology Faculty Publications and Presentations. 99. https://pdxscholar.library.pdx.edu/anth_fac/99 This Report is brought to you for free and open access. It has been accepted for inclusion in Anthropology Faculty Publications and Presentations by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. National Park Service U.S. Department of the Interior Wrangell-St. Elias National Park and Preserve Yakutat Tlingit and Wrangell-St. Elias National Park and Preserve: An Ethnographic Overview and Assessment Douglas Deur, Ph.D. Thomas Thornton, Ph.D. Rachel Lahoff, M.A. Jamie Hebert, M.A. 2015 Cover photos: Mount St. Elias / Was'ei Tashaa (courtesy Wikimedia Commons); Mount St. Elias Dancers (courtesy Yakutat Tlingit Tribe / Bert Adams Sr.) Yakutat Tlingit and Wrangell-St. Elias National Park and Preserve: An Ethnographic Overview and Assessment 2015 Douglas Deur, Thomas Thornton, Rachel Lahoff, and Jamie Hebert Portland State University Department of Anthropology United States Department of the Interior National Park Service Wrangell-St. -

Environmental and Cultural Overview of the Yukon Flats Region Prepared By: Kevin Bailey, USFWS Archaeologist Date: 2/12/2015 In
Environmental and Cultural Overview of the Yukon Flats Region Prepared by: Kevin Bailey, USFWS Archaeologist Date: 2/12/2015 Introduction With a substantial population of Native people residing in their traditional homeland and living a modern traditional lifestyle, the Yukon Flats Refuge and all of the Alaskan Interior is a dynamic and living cultural landscape. The land, people, and wildlife form a tight, interrelated web of relationships extending thousands of years into the past. Natural features and human created “sites” form a landscape of meaning to the modern residents. The places and their meanings are highly relevant to modern residents, not just for people and culture but for the land. To many Gwich’in people culture is not distinct from their homeland. Although only minimally discussed in this overview, this dynamic living cultural landscape should be considered and discussed when writing about this area. Environmental Setting Containing the largest interior basin in Alaska, the Yukon Flats Refuge encompasses over 11 million acres of land in east central Alaska. Extending roughly 220 miles east-west along the Arctic Circle, the refuge lies between the Brooks Range to the north, and the White-Crazy Mountains to the south. The pipeline corridor runs along the refuge’s western boundary while the eastern boundary extends within 30 miles of the Canadian border. The Yukon River bisects the refuge, creating the dominant terrain. As many as 40,000 lakes, ponds, and streams may occur on the refuge, most concentrated in the flood plain along the Yukon and other rivers. Upland terrain, where lakes are less abundant, is the source of important drainage systems. -
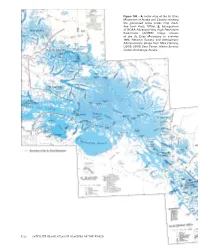
A, Index Map of the St. Elias Mountains of Alaska and Canada Showing the Glacierized Areas (Index Map Modi- Fied from Field, 1975A)
Figure 100.—A, Index map of the St. Elias Mountains of Alaska and Canada showing the glacierized areas (index map modi- fied from Field, 1975a). B, Enlargement of NOAA Advanced Very High Resolution Radiometer (AVHRR) image mosaic of the St. Elias Mountains in summer 1995. National Oceanic and Atmospheric Administration image from Mike Fleming, USGS, EROS Data Center, Alaska Science Center, Anchorage, Alaska. K122 SATELLITE IMAGE ATLAS OF GLACIERS OF THE WORLD St. Elias Mountains Introduction Much of the St. Elias Mountains, a 750×180-km mountain system, strad- dles the Alaskan-Canadian border, paralleling the coastline of the northern Gulf of Alaska; about two-thirds of the mountain system is located within Alaska (figs. 1, 100). In both Alaska and Canada, this complex system of mountain ranges along their common border is sometimes referred to as the Icefield Ranges. In Canada, the Icefield Ranges extend from the Province of British Columbia into the Yukon Territory. The Alaskan St. Elias Mountains extend northwest from Lynn Canal, Chilkat Inlet, and Chilkat River on the east; to Cross Sound and Icy Strait on the southeast; to the divide between Waxell Ridge and Barkley Ridge and the western end of the Robinson Moun- tains on the southwest; to Juniper Island, the central Bagley Icefield, the eastern wall of the valley of Tana Glacier, and Tana River on the west; and to Chitistone River and White River on the north and northwest. The boundar- ies presented here are different from Orth’s (1967) description. Several of Orth’s descriptions of the limits of adjacent features and the descriptions of the St. -

The Effect of Snowmobile Trails on Coyote Movements Within Lynx Home Ranges
Research Article The Effect of Snowmobile Trails on Coyote Movements Within Lynx Home Ranges JAY A. KOLBE,1 Montana Department of Fish, Wildlife, and Parks, Seeley Lake, MT 59868, USA JOHN R. SQUIRES, United States Forest Service, Rocky Mountain Research Station, Box 8089, Missoula, MT 59807, USA DANIEL H. PLETSCHER, Wildlife Biology Program, Department of Ecosystem and Conservation Sciences, University of Montana, Missoula, MT 59812, USA LEONARD F. RUGGIERO, United States Forest Service, Rocky Mountain Research Station, Box 8089, Missoula, MT 59807, USA ABSTRACT Coyotes (Canis latrans) and Canada lynx (Lynx canadensis) are sympatric throughout much of the lynx’s southern range. Researchers and managers have suggested that the presence of compacted snowmobile trails may allow coyotes to access lynx habitat from which they were previously excluded by deep, unconsolidated snow. This could then allow coyotes to more effectively compete with lynx for snowshoe hares (Lepus americanus), the lynx’s primary prey. We investigated how coyotes interacted with compacted snowmobile trails by conducting carnivore track surveys and by snow tracking adult coyotes (4 M, 8 F) in areas of western Montana, USA, with both documented lynx presence and recreational snowmobile use. Coyotes remained in lynx habitat having deep snow throughout the winter months. They used compacted snowmobile trails for 7.69% of their travel distance and traveled on them for a median distance of 124 m. Coyotes used compacted forest roads (5.66% of total travel) and uncompacted forest roads (4.62% of total travel) similarly. Coyotes did not travel closer to compacted snowmobile trails than random expectation (coyote x¯ distance from compacted trails ¼ 368 m, random expectation ¼ 339 m) and the distance they traveled from these trails did not vary with daily, monthly, or yearly changes in snow supportiveness or depth. -

Glaciochemical Records from the Saint Elias Mountains, Yukon, Canada Kaplan B
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Fall 2005 Glaciochemical records from the Saint Elias Mountains, Yukon, Canada Kaplan B. Yalcin University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Yalcin, Kaplan B., "Glaciochemical records from the Saint Elias Mountains, Yukon, Canada" (2005). Doctoral Dissertations. 293. https://scholars.unh.edu/dissertation/293 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. GLACIOCHEMICAL RECORDS FROM THE ST. ELIAS MOUNTAINS, YUKON, CANADA BY KAPLAN B. YALCIN B.S. with honors. University of Missouri, 1998 M.S., University of New Hampshire, 2001 DISSERTATION Submitted to the University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of Doctor of Philosophy in Earth and Environmental Science September 2005 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 3183907 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion.