Molecular Phylogeny of Selected Species of the Order Dinophysiales (Dinophyceae)—Testing the Hypothesis of a Dinophysioid Radiation1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

182-188 Enhanced Chlorophyll a and Primary Production in the Northern
Author version: Mar. Biol. Res., vol.8; 2012; 182-188 Enhanced chlorophyll a and primary production in the northern Arabian Sea during the spring intermonsoon due to green Noctiluca (N. scintillans) bloom N. V. Madhua,*, R. Jyothibabua, P. A. Maheswaranb, K. A. Jayaraja, C.T. Achuthankuttya aNational Institute of Oceanography, Regional Centre, Kochi -18, India bNaval Physical Oceanographic Laboratory, Kochi - 21, India Abstract The surface waters of the northeastern Arabian Sea sustained relatively high chlorophyll a (av. 0.81 ± 0.80 mgm-3) and primary production (av. 29.5 ± 23.6 mgC m-3d-1) during the early spring intermonsoon 2000. This was caused primarily by a thick patch of algal bloom spread over a vast area between 17° to 21°N and 66 to 70°E. Satellite images showed exceptionally high concentration of chlorophyll a in the bloom areas, representing the annually occurring ‘spring blooms’ during February-March. The causative organism of the bloom was the dinoflagellate, Noctiluca scintillans Macartney (synonym Noctiluca miliaris Suriray, Dinophyceae: Noctilucidea), symbiotically associated with an autotrophic prasinophyte Pedinomonas noctilucae. The symbiosis between N. scintillans and P. noctilucae is likely responsible for their explosive growth (av. 3 million cells L-1) over an extensive area making the northeastern Arabian Sea highly productive (av. 607 ± 338 mg Cm-3d-1) even during an oligotrophic period such as spring intermonsoon. Key words: - Chlorophyll a; Algal bloom; Noctiluca scintillans, Pedinomonas noctilucae; Spring intermonsoon *Email of the corresponding author - [email protected] 2 Introduction The Arabian Sea (AS hereafter) is one of the most productive regions in the Indian Ocean (Madhupratap et al., 1996), exhibiting a bimodal temperature cycles annually, with lows during winter (northeast monsoon - NEM) and summer (southwest monsoon - SWM) seasons. -

Check List 15 (5): 951–963
15 5 ANNOTATED LIST OF SPECIES Check List 15 (5): 951–963 https://doi.org/10.15560/15.5.951 Dinoflagellates in tropical estuarine waters from the Maraú River, Camamu Bay, northeastern Brazil Caio Ceza da Silva Nunes1, Sylvia Maria Moreira Susini-Ribeiro1, 2, Kaoli Pereira Cavalcante3 1 Mestrado em Sistemas Aquáticos Tropicais, Universidade Estadual de Santa Cruz, Rodovia Jorge Amado, km 16, Salobrinho, 45662090 Ilhéus, BA, Brazil. 2 Universidade Estadual de Santa Cruz, Rodovia Jorge Amado, km 16, Salobrinho, 45662090 Ilhéus, BA, Brazil. 3 Universidade Estadual Vale do Acaraú, Avenida da Universidade, 850, Campus da Betânia, Betânia, 62040370, Sobral, CE, Brazil. Corresponding author: Caio Ceza da Silva Nunes, [email protected] Abstract Dinoflagellates display great diversity in tropical regions and play an important role in the complex microbial food webs of marine and brackish environments. The goal of this study is to identify planktonic dinoflagellates and their distribution in the estuary of the Maraú River, Camamu Bay, state of Bahia, in a region with increasing use of shellfish farming. Samples were carried out monthly from August 2006 to July 2007 at four stations along the estuary. Plankton was sampled with a 20 μm mesh net. We identified 20 dinoflagellate species. The greatest species richness was ob- served in the genera Protoperidinium (five spp.), Tripos (four spp.), and Prorocentrum (three spp.). Based on literature, six species were classified as potentially harmful: Akashiwo sanguinea, Dinophysis caudata, Gonyaulax spinifera, Prorocentrum micans, Scrippsiella cf. acuminata, and Tripos furca. Protoperidinium venustum was recorded for the first time in coastal waters of Bahia. Keywords Brackish water, Dinophyta, distribution, potentially harmful species, taxonomy. -

Genomics Reveals Alga-Associated Cyanobacteria Hiding in Plain Sight COMMENTARY John M
COMMENTARY Genomics reveals alga-associated cyanobacteria hiding in plain sight COMMENTARY John M. Archibalda,b,1 Cyanobacteria occupy a special place in the pantheon of prokaryotic life. It is in the ancestors of these ubiquitous microbes that oxygenic photosynthesis first evolved more than 2 billion y ago (1), and it is from endosymbiotic cyanobacteria that the plastids (chloro- plasts) of plants and algae are derived (2). Modern-day cyanobacteria are diverse in form and function; they in- clude coccoid marine picoplankton such as Prochloro- coccus (3), freshwater biofilm-forming genera [e.g., Gloeomargarita (4)], and filamentous taxa capable of fix- ing nitrogen [e.g., Nostoc (5)]. In PNAS, Nakayama et al. (6) add an exciting chapter to the story of cyanobacterial diversity. The authors describe the genome sequence of a cyanobacterium living ectosymbiotically on an eye- catching dinoflagellate named Ornithocercus magnifi- cus. Their results provide insight into the nature of an enigmatic symbiotic relationship and reveal the exis- tence of a cryptic, globally distributed cyanobacterial lineage that has until now gone unappreciated. Ornithocercus is indeed magnificent, even by di- Fig. 1. Light micrograph of an Ornithocercus noflagellate standards. The surface of this heterotro- dinoflagellate. The ectosymbiotic OmCyn cyanobacteria phic marine protist is decorated with crown-shaped, are visible within an extracellular chamber on the cellulosic outcroppings that extend from the cell body “upper” crown of the cell. Image courtesy of Takuro Nakayama (Tohoku University, Sendai, Japan). in different directions (Fig. 1) (7). The “upper” crown forms an extracellular chamber in which autofluores- cent cyanobacteria reside, and microscopic evidence genome of the organism, which they dubbed “OmCyn.” suggests that the bacteria can be vertically transmitted In a preliminary phylogenetic analysis of the 16S ribo- from mother to daughter chambers during host cell somal RNA (rRNA) gene, OmCyn was found to branch division (8). -

The Plankton Lifeform Extraction Tool: a Digital Tool to Increase The
Discussions https://doi.org/10.5194/essd-2021-171 Earth System Preprint. Discussion started: 21 July 2021 Science c Author(s) 2021. CC BY 4.0 License. Open Access Open Data The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data Clare Ostle1*, Kevin Paxman1, Carolyn A. Graves2, Mathew Arnold1, Felipe Artigas3, Angus Atkinson4, Anaïs Aubert5, Malcolm Baptie6, Beth Bear7, Jacob Bedford8, Michael Best9, Eileen 5 Bresnan10, Rachel Brittain1, Derek Broughton1, Alexandre Budria5,11, Kathryn Cook12, Michelle Devlin7, George Graham1, Nick Halliday1, Pierre Hélaouët1, Marie Johansen13, David G. Johns1, Dan Lear1, Margarita Machairopoulou10, April McKinney14, Adam Mellor14, Alex Milligan7, Sophie Pitois7, Isabelle Rombouts5, Cordula Scherer15, Paul Tett16, Claire Widdicombe4, and Abigail McQuatters-Gollop8 1 10 The Marine Biological Association (MBA), The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK. 2 Centre for Environment Fisheries and Aquacu∑lture Science (Cefas), Weymouth, UK. 3 Université du Littoral Côte d’Opale, Université de Lille, CNRS UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Wimereux, France. 4 Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. 5 15 Muséum National d’Histoire Naturelle (MNHN), CRESCO, 38 UMS Patrinat, Dinard, France. 6 Scottish Environment Protection Agency, Angus Smith Building, Maxim 6, Parklands Avenue, Eurocentral, Holytown, North Lanarkshire ML1 4WQ, UK. 7 Centre for Environment Fisheries and Aquaculture Science (Cefas), Lowestoft, UK. 8 Marine Conservation Research Group, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. 9 20 The Environment Agency, Kingfisher House, Goldhay Way, Peterborough, PE4 6HL, UK. 10 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, AB11 9DB, UK. -

De Rijk, L?, Caers, A,, Van De Peer, Y. & De Wachter, R. 1998. Database
BLANCHARD & HICKS-THE APICOMPLEXAN PLASTID 375 De Rijk, l?, Caers, A,, Van de Peer, Y. & De Wachter, R. 1998. Database gorad, L. & Vasil, I. K. (ed.), Cell Culture and Somatic Cell Genetics on the structure of large ribosomal subunit RNA. Nucl. Acids. Rex, of Plants, Vol7A: The molecular biology of plastids. Academic Press, 26: 183- 186. San Diego. p. 5-53. Deveraux, J., Haeberli, l? & Smithies, 0. 1984. A comprehensive set of Palmer, J. D. & Delwiche, C. E 1996. Second-hand chloroplasts and sequence analysis programs for the VAX. Nucl. Acids. Rex, 12:387-395. the case of the disappearing nucleus. Proc. Natl. Acad. Sci. USA, 93: Eaga, N. & Lang-Unnasch, N. 1995. Phylogeny of the large extrachro- 7432-7435. mosomal DNA of organisms in the phylum Apicomplexa. J. Euk. Popadic, A,, Rusch, D., Peterson, M., Rogers, B. T. & Kaufman, T. C. Microbiol,, 42:679-684. 1996. Origin of the arthropod mandible. Nature, 380:395. Fichera, M. E. & Roos, D. S. 1997. A plastid organelle as a drug target Preiser, l?, Williamson, D. H. & Wilson, R. J. M. 1995. Transfer-RNA in apicomplexan parasites. Nature, 390:407-409. genes transcribed from the plastid-like DNA of Plasmodium falci- Gardner, M. J., Williamson, D. H. & Wilson, R. J. M. 1991. A circular parum. Nucl. Acids Res., 23:4329-4336. DNA in malaria parasites encodes an RNA polymerase like that of Reith. M. & Munholland, J. 1993. A high-resolution gene map of the prokaryotes and chloroplasts. Mol. Biochem. Parasitiol., 44: 1 15-123. chloroplast genome of the red alga Porphyra purpurea. Plant Cell, Gardner, M. -

Checklist of Mediterranean Free-Living Dinoflagellates Institutional Rate: € 938,-/Approx
Botanica Marina Vol. 46,2003, pp. 215-242 © 2003 by Walter de Gruyter • Berlin ■ New York Subscriptions Botanica Marina is published bimonthly. Annual subscription rate (Volume 46,2003) Checklist of Mediterranean Free-living Dinoflagellates Institutional rate: € 938,-/approx. SFr1 50 1 in the US and Canada US $ 938,-. Individual rate: € 118,-/approx. SFr 189,-; in the US and Canada US $ 118,-. Personal rates apply only when copies are sent to F. Gómez a private address and payment is made by a personal check or credit card. Personal subscriptions must not be donated to a library. Single issues: € 178,-/approx. SFr 285,-. All prices exclude postage. Department of Aquatic Biosciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657, Japan, [email protected] Orders Institutional subscription orders should be addressed to the publishers orto your usual subscription agent. Individual subscrip tion orders must be sent directly to one of the addresses indicated below. The Americas: An annotated checklist of the free-living dinoflagellates (Dinophyceae) of the Mediterranean Sea, based on Walter de Gruyter, Inc., 200 Saw Mill River Road, Hawthorne, N.Y. 10532, USA, Tel. 914-747-0110, Fax 914-747-1326, literature records, is given. The distribution of 673 species in 9 Mediterranean sub-basins is reported. The e-mail: [email protected]. number of taxa among the sub-basins was as follows: Ligurian (496 species), Balear-Provençal (360), Adri Australia and New Zealand: atic (322), Tyrrhenian (284), Ionian (283), Levantine (268), Aegean (182), Alborán (179) and Algerian Seas D. A. Information Services, 648 Whitehorse Road, P.O. -

Mixotrophic Protists Among Marine Ciliates and Dinoflagellates: Distribution, Physiology and Ecology
FACULTY OF SCIENCE UNIVERSITY OF COPENHAGEN PhD thesis Woraporn Tarangkoon Mixotrophic Protists among Marine Ciliates and Dinoflagellates: Distribution, Physiology and Ecology Academic advisor: Associate Professor Per Juel Hansen Submitted: 29/04/10 Contents List of publications 3 Preface 4 Summary 6 Sammenfating (Danish summary) 8 สรุป (Thai summary) 10 The sections and objectives of the thesis 12 Introduction 14 1) Mixotrophy among marine planktonic protists 14 1.1) The role of light, food concentration and nutrients for 17 the growth of marine mixotrophic planktonic protists 1.2) Importance of marine mixotrophic protists in the 20 planktonic food web 2) Marine symbiont-bearing dinoflagellates 24 2.1) Occurrence of symbionts in the order Dinophysiales 24 2.2) The spatial distribution of symbiont-bearing dinoflagellates in 27 marine waters 2.3) The role of symbionts and phagotrophy in dinoflagellates with symbionts 28 3) Symbiosis and mixotrophy in the marine ciliate genus Mesodinium 30 3.1) Occurrence of symbiosis in Mesodinium spp. 30 3.2) The distribution of marine Mesodinium spp. 30 3.3) The role of symbionts and phagotrophy in marine Mesodinium rubrum 33 and Mesodinium pulex Conclusion and future perspectives 36 References 38 Paper I Paper II Paper III Appendix-Paper IV Appendix-I Lists of publications The thesis consists of the following papers, referred to in the synthesis by their roman numerals. Co-author statements are attached to the thesis (Appendix-I). Paper I Tarangkoon W, Hansen G Hansen PJ (2010) Spatial distribution of symbiont-bearing dinoflagellates in the Indian Ocean in relation to oceanographic regimes. Aquat Microb Ecol 58:197-213. -

Bacterial and Archaeal Symbioses with Protists, Current Biology (2021), J.Cub.2021.05.049
Please cite this article in press as: Husnik et al., Bacterial and archaeal symbioses with protists, Current Biology (2021), https://doi.org/10.1016/ j.cub.2021.05.049 ll Review Bacterial and archaeal symbioses with protists Filip Husnik1,2,*, Daria Tashyreva3, Vittorio Boscaro2, Emma E. George2, Julius Lukes3,4, and Patrick J. Keeling2,* 1Okinawa Institute of Science and Technology, Okinawa, 904-0495, Japan 2Department of Botany, University of British Columbia, Vancouver, V6T 1Z4, Canada 3Institute of Parasitology, Biology Centre, Czech Academy of Sciences, Ceske Budejovice, 370 05, Czech Republic 4Faculty of Science, University of South Bohemia, Ceske Budejovice, 370 05, Czech Republic *Correspondence: fi[email protected] (F.H.), [email protected] (P.J.K.) https://doi.org/10.1016/j.cub.2021.05.049 SUMMARY Most of the genetic, cellular, and biochemical diversity of life rests within single-celled organisms—the pro- karyotes (bacteria and archaea) and microbial eukaryotes (protists). Very close interactions, or symbioses, between protists and prokaryotes are ubiquitous, ecologically significant, and date back at least two billion years ago to the origin of mitochondria. However, most of our knowledge about the evolution and functions of eukaryotic symbioses comes from the study of animal hosts, which represent only a small subset of eukary- otic diversity. Here, we take a broad view of bacterial and archaeal symbioses with protist hosts, focusing on their evolution, ecology, and cell biology, and also explore what functions (if any) the symbionts provide to their hosts. With the immense diversity of protist symbioses starting to come into focus, we can now begin to see how these systems will impact symbiosis theory more broadly. -

The Windblown: Possible Explanations for Dinophyte DNA
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.07.242388; this version posted August 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The windblown: possible explanations for dinophyte DNA in forest soils Marc Gottschlinga, Lucas Czechb,c, Frédéric Mahéd,e, Sina Adlf, Micah Dunthorng,h,* a Department Biologie, Systematische Botanik und Mykologie, GeoBio-Center, Ludwig- Maximilians-Universität München, D-80638 Munich, Germany b Computational Molecular Evolution Group, Heidelberg Institute for Theoretical Studies, D- 69118 Heidelberg, Germany c Department of Plant Biology, Carnegie Institution for Science, Stanford, CA 94305, USA d CIRAD, UMR BGPI, F-34398, Montpellier, France e BGPI, Université de Montpellier, CIRAD, IRD, Montpellier SupAgro, Montpellier, France f Department of Soil Sciences, College of Agriculture and Bioresources, University of Saskatchewan, Saskatoon, S7N 5A8, SK, Canada g Eukaryotic Microbiology, Faculty of Biology, Universität Duisburg-Essen, D-45141 Essen, Germany h Centre for Water and Environmental Research (ZWU), Universität Duisburg-Essen, D- 45141 Essen, Germany Running title: Dinophytes in soils Correspondence M. Dunthorn, Eukaryotic Microbiology, Faculty of Biology, Universität Duisburg-Essen, Universitätsstrasse 5, D-45141 Essen, Germany Telephone number: +49-(0)-201-183-2453; email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.08.07.242388; this version posted August 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
Diversity.Pdf
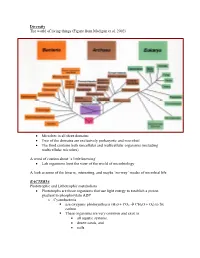
Diversity The world of living things (Figure from Madigan et al. 2002) • Microbes in all three domains • Two of the domains are exclusively prokaryotic and microbial • The third contains both unicellular and multicellular organisms (including multicellular microbes) A word of caution about ‘a little knowing’ • Lab organisms limit the view of the world of microbiology A look at some of the bizarre, interesting, and maybe ‘no-way’ modes of microbial life BACTERIA Phototrophic and Lithotrophic metabolism • Phototrophs are those organisms that use light energy to establish a proton gradient to phosphorylate ADP o Cyanobacteria use oxygenic photosynthesis (H20 + CO2 Æ CH2O + O2) to fix carbon. These organisms are very common and exist in • all aquatic systems, • desert sands, and • soils. Many have the ability to fix elemental N2 into NH3 and this only enhances their ability to survive in diverse habitats Why is this important? (Table-Figures from Madigan et al. 2002) Figure 14.38 o Phototrophic bacteria Use anoxygenic photosynthesis (H2S + CO2 Æ CH2O + S2) to fix carbon into cell stuff Primarily found in restricted habitats • H2S must be available • Light must be available • Located in ‘bands in lakes and near the surface of muddy environments Some may grow chemoorganotrophically in the dark Some may grow photoheterotrophically (Figures from Madigan et al. 2002) • Chemolithotrophs o Organisms that obtain energy from oxidation of inorganic compounds o Most can fix CO2 into cell stuff using the Calvin Cycle (that is the one green plants use) o Many can obtain reduced coenzymes by operating electron transport chains in reverse. Nitrifying bacteria • Grow at the expense of reduced inorganic nitrogen and all oxidations are aerobic. -

VII EUROPEAN CONGRESS of PROTISTOLOGY in Partnership with the INTERNATIONAL SOCIETY of PROTISTOLOGISTS (VII ECOP - ISOP Joint Meeting)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/283484592 FINAL PROGRAMME AND ABSTRACTS BOOK - VII EUROPEAN CONGRESS OF PROTISTOLOGY in partnership with THE INTERNATIONAL SOCIETY OF PROTISTOLOGISTS (VII ECOP - ISOP Joint Meeting) Conference Paper · September 2015 CITATIONS READS 0 620 1 author: Aurelio Serrano Institute of Plant Biochemistry and Photosynthesis, Joint Center CSIC-Univ. of Seville, Spain 157 PUBLICATIONS 1,824 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Use Tetrahymena as a model stress study View project Characterization of true-branching cyanobacteria from geothermal sites and hot springs of Costa Rica View project All content following this page was uploaded by Aurelio Serrano on 04 November 2015. The user has requested enhancement of the downloaded file. VII ECOP - ISOP Joint Meeting / 1 Content VII ECOP - ISOP Joint Meeting ORGANIZING COMMITTEES / 3 WELCOME ADDRESS / 4 CONGRESS USEFUL / 5 INFORMATION SOCIAL PROGRAMME / 12 CITY OF SEVILLE / 14 PROGRAMME OVERVIEW / 18 CONGRESS PROGRAMME / 19 Opening Ceremony / 19 Plenary Lectures / 19 Symposia and Workshops / 20 Special Sessions - Oral Presentations / 35 by PhD Students and Young Postdocts General Oral Sessions / 37 Poster Sessions / 42 ABSTRACTS / 57 Plenary Lectures / 57 Oral Presentations / 66 Posters / 231 AUTHOR INDEX / 423 ACKNOWLEDGMENTS-CREDITS / 429 President of the Organizing Committee Secretary of the Organizing Committee Dr. Aurelio Serrano -

Scrippsiella Trochoidea (F.Stein) A.R.Loebl
MOLECULAR DIVERSITY AND PHYLOGENY OF THE CALCAREOUS DINOPHYTES (THORACOSPHAERACEAE, PERIDINIALES) Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Fakultät für Biologie der Ludwig-Maximilians-Universität München zur Begutachtung vorgelegt von Sylvia Söhner München, im Februar 2013 Erster Gutachter: PD Dr. Marc Gottschling Zweiter Gutachter: Prof. Dr. Susanne Renner Tag der mündlichen Prüfung: 06. Juni 2013 “IF THERE IS LIFE ON MARS, IT MAY BE DISAPPOINTINGLY ORDINARY COMPARED TO SOME BIZARRE EARTHLINGS.” Geoff McFadden 1999, NATURE 1 !"#$%&'(&)'*!%*!+! +"!,-"!'-.&/%)$"-"!0'* 111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111 2& ")3*'4$%/5%6%*!+1111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111 7! 8,#$0)"!0'*+&9&6"*,+)-08!+ 111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111 :! 5%*%-"$&0*!-'/,)!0'* 11111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111 ;! "#$!%"&'(!)*+&,!-!"#$!'./+,#(0$1$!2! './+,#(0$1$!-!3+*,#+4+).014!1/'!3+4$0&41*!041%%.5.01".+/! 67! './+,#(0$1$!-!/&"*.".+/!1/'!4.5$%"(4$! 68! ./!5+0&%!-!"#$!"#+*10+%,#1$*10$1$! 69! "#+*10+%,#1$*10$1$!-!5+%%.4!1/'!$:"1/"!'.;$*%."(! 6<! 3+4$0&41*!,#(4+)$/(!-!0#144$/)$!1/'!0#1/0$! 6=! 1.3%!+5!"#$!"#$%.%! 62! /0+),++0'* 1111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111111<=!