A Thesis Entitled the Effects of Radial Core Decompression on Lunate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
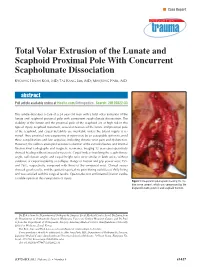
Total Volar Extrusion of the Lunate and Scaphoid Proximal Pole with Concurrent Scapholunate Dissociation
n Case Report Total Volar Extrusion of the Lunate and Scaphoid Proximal Pole With Concurrent Scapholunate Dissociation KYOUNG HWAN KOH, MD; TAE KANG LIM, MD; MIN JONG PARK, MD abstract Full article available online at Healio.com/Orthopedics. Search: 20120822-33 This article describes a case of a 24-year-old man with a total volar extrusion of the lunate and scaphoid proximal pole with concurrent scapholunate dissociation. The viability of the lunate and the proximal pole of the scaphoid are at high risk in this type of injury. Scaphoid nonunion, avascular necrosis of the lunate and proximal pole of the scaphoid, and carpal instability are inevitable unless the blood supply is re- stored. Thus, proximal row carpectomy at injury may be an acceptable option to avoid these complications and late sequelae, including chronic wrist pain and dysfunction. However, the authors attempted accurate reduction of the extruded bones and internal fixation.Final radiographs and magnetic resonance imaging 12 years postoperatively showed healing without avascular necrosis. Carpal indices involving the scapholunate angle, radiolunate angle, and carpal height ratio were similar in both wrists without evidence of carpal instability or collapse. Range of motion and grip power were 75% and 76%, respectively, compared with those of the uninjured wrist. Clinical scores showed good results, and the patient reported no pain during activities of daily living and was satisfied with his surgical results. Open reduction and internal fixation can be a viable option in this rare pattern of injury. Figure: Intraoperative photograph showing the me- dian nerve (arrow), which was compressed by the displaced lunate (asterisk) and scaphoid fracture. -

Lunate Flattening in Rheumatoid Wrists
Central Annals of Orthopedics & Rheumatology Research Article *Corresponding author Ryogo Nakamura, Nagoya Hand Center, Chunichi Hospital, 3-12-3 Marunouchi, Naka-ku, Nagoya 460- Lunate Flattening in 0002, Japan, Email: Submitted: 04 February 2014 Accepted: 28 May 2014 Rheumatoid Wrists Published: 30 May 2014 Ryogo Nakamura1, Satoshi Niwa1, Sayako Takahashi1, Etushiro Copyright © 2014 Nakamura et al. Naka1 and Masahiro Tatebe2* 1Nagoya Hand Center Chunichi Hospital, Nagoya, Japan OPEN ACCESS 2Hand and Microsurgery Center Anjo Kosei Hospital, Japan Abstract Lunate flattening was studied in 36 patients (62 wrists) with rheumatoid arthritis (RA) who were age- and gender-matched to people with normal wrists. The carpal height ratio (CHR) and lunate compression ratio (LCR; lunate height/ lunate diameter×100, Ståhl index) were measured from wrist radiographs of both groups and the lunate flattening ratio (LFR) was calculated by dividing the LCR by the CHR. The LFR reflects lunate flattening compared to carpal collapse. Twenty-four wrists underwent MRI study. T1-weighted images were evaluated for bone necrosis of the lunate. Results: the normal LFR value obtained from normal wrists ranged from 79.9 to 113.5 (mean±2SD). Lunates with RA could be classified into three groups by LFR values- lunates with a LFR above 113.5 (10 wrists, 16%), lunates with a LFR within the normal range (34 wrists, 55%), and lunates with a LFR lower than 79.9 (18 wrists, 29%). Twelve (50%) of the 24 wrists that underwent MRI study showed a low signal of the lunate in T1-weighted images showing osteonecrosis. Although a satisfactory cor- relation was not confirmed between a LFR value and a low signal on T1-weighted im- ages, lunate avascular necrosis was suspected to play a role in flattening of the lunate. -
The Appendicular Skeleton the Appendicular Skeleton
The Appendicular Skeleton Figure 8–1 The Appendicular Skeleton • Allows us to move and manipulate objects • Includes all bones besides axial skeleton: – the limbs – the supportive girdles 1 The Pectoral Girdle Figure 8–2a The Pectoral Girdle • Also called the shoulder girdle • Connects the arms to the body • Positions the shoulders • Provides a base for arm movement 2 The Clavicles Figure 8–2b, c The Clavicles • Also called collarbones • Long, S-shaped bones • Originate at the manubrium (sternal end) • Articulate with the scapulae (acromial end) The Scapulae Also called shoulder blades Broad, flat triangles Articulate with arm and collarbone 3 The Scapula • Anterior surface: the subscapular fossa Body has 3 sides: – superior border – medial border (vertebral border) – lateral border (axillary border) Figure 8–3a Structures of the Scapula Figure 8–3b 4 Processes of the Glenoid Cavity • Coracoid process: – anterior, smaller •Acromion: – posterior, larger – articulates with clavicle – at the acromioclavicular joint Structures of the Scapula • Posterior surface Figure 8–3c 5 Posterior Features of the Scapula • Scapular spine: – ridge across posterior surface of body • Separates 2 regions: – supraspinous fossa – infraspinous fossa The Humerus Figure 8–4 6 Humerus • Separated by the intertubercular groove: – greater tubercle: • lateral • forms tip of shoulder – lesser tubercle: • anterior, medial •Head: – rounded, articulating surface – contained within joint capsule • Anatomical neck: – margin of joint capsule • Surgical neck: – the narrow -

Ulnar Variance and the Shape of the Lunate Bone a Radiological Investigation
ULNAR VARIANCE AND THE SHAPE OF THE LUNATE BONE A RADIOLOGICAL INVESTIGATION A. H. SCHUURMAN1, M. MAAS2, P. F. DIJKSTRA2, J. M. G. KAUER3 The authors designed a study to test the hypothesis Ulnar variance is the roentgenographic difference that the length of the ulna might affect the shape of of the length of the ulna relative to the radius. the lunate bone because of long-term molding during Positive ulna variance or ulna plus refers to an ulna life. This might then be useful to predict the presence longer than the radius, while the opposite is true in or absence of a dynamic or static ulna plus by the the ulna minus or negative ulnar variance. shape of the lunate bone. Obermann (6) correlated lunate shape and ulnar In a prospective study, posterior-anterior wrist xrays length in 68 patients. Although he found a 13% were taken in a standard fashion in 68 patients with a mean age of 34.5 years. Dominance, grip strength, incidence of type 1, 62% type 2 and 25% type 3, he ulnar variance and the shape of the lunate were found no correlation between type 1 and ulna recorded. length (even distribution in all). Type 2 was found Lunate shape, type 1, which is the least molded, was less often in the ulna minus group and type 3 was seen most frequently on both the left and right side most often found in the ulna plus group. Unfortu- and did not correlate with the dominant side. The nately no statistical analysis was performed. -

Multifocal Avascular Necrosis of the Lunate and Triquetrum in a Child
ISSN: 2469-5726 Roberts et al. J Rheum Dis Treat 2018, 4:063 DOI: 10.23937/2469-5726/1510063 Volume 4 | Issue 2 Journal of Open Access Rheumatic Diseases and Treatment CASE REPORT Multifocal Avascular Necrosis of the Lunate and Triquetrum in a Child with Systemic Lupus Erythematosus Roberts DC1*, Jester A1, Southwood T2, Johnson K3 and Oestreich K1 1 Department of Hand, Plastic and Reconstructive Surgery, Birmingham Children’s Hospital, UK Check for 2Department of Rheumatology, Birmingham Children’s Hospital, UK updates 3Department of Radiology, Birmingham Children’s Hospital, UK *Corresponding author: Darren Roberts, Department of Hand, Plastic and Reconstructive Surgery, Birmingham Children’s Hospital, UK, E-mail: [email protected] We present, to our knowledge, the first case of syn- Abstract chronous lunate and triquetral AVN in a child with SLE. Avascular necrosis is a known complication of systemic lu- The aetiology and management of carpal AVN in child- pus erythematosus. We report an unusual case of avas- cular necrosis affecting both the lunate and triquetrum in a hood SLE are also discussed based on the available lit- child with this condition. Vasculitis, synovitis and IgM anti- erature. cardiolipin antibodies were probable predisposing factors. The use of arthroscopic synovial debridement improves Case Report symptoms even in the presence of carpal chondromalacia and potentially delays the need for salvage surgery. A 14-year-old girl, diagnosed with SLE five years pre- viously, presented with a two-year history of non-trau- Introduction matic pain and stiffness of her right dominant wrist. She was able to write and self-care, however she was unable Avascular necrosis (AVN) of the carpus typically af- to partake in hand-held ball and racquet sports at school. -

Intraosseous Ganglion Cyst of the Lunate Bone: Two Case Reports with Literature Review Georgi P
ISSN: 2474-7564 Case Report Mathews Journal of Orthopedics Intraosseous Ganglion Cyst of The Lunate Bone: Two Case Reports with Literature Review Georgi P. Georgiev1, Iva N. Dimitrova2, Alexandar Iliev3, Georgi Kotov3, Boycho Landzhov3 1Department of Orthopaedics and Traumatology, University Hospital Queen Giovanna - ISUL, Medical University of Sofia, Bulgaria. 2Department of Cardiology, University Hospital St. Ekaterina, Medical University of Sofia, Bulgaria. 3Department of Anatomy, Histology and Embryology, Medical University of Sofia, Bulgaria. Corresponding Author: Georgi P. Georgiev, Department of Orthopaedics and Traumatology, University Hospital Queen Giovanna -ISUL, Medical University-Sofia, 8 Bialo more str., BG 1527, Sofia, Bulgaria, Tel: +359884 493523; Email: [email protected] Received Date: 04 Dec 2016 Copyright © 2016 Georgiev GP Accepted Date: 13 Dec 2016 Citation: Georgiev GP, Dimitrova IN, Iliev A, Kotov G, et al. Published Date: 14 Dec 2016 (2016). Intraosseous Ganglion Cyst of The Lunate Bone: Two Case Reports With Literature Review. M J Orth. 1(2): 012. ABSTRACT Intraosseous ganglia of the carpal bones are infrequent, benign, non-neoplastic bone lesions, which are observed pre- dominantly in young and middle-aged adults. The most commonly affected bones are the lunate and scaphoid, followed by the capitate, triquetrum and trapezoid bones. Carpal intraosseous ganglia are uncommon causes of chronic wrist pain. They have a broad and complex differential diagnosis based on various imaging modalities and histological examination. Treatment of such lesions involves several techniques and is associated with complete cure of the symptoms and low rate of recurrence. In this study, we present two cases with an intraosseous ganglion cyst of the lunate bone. -

Secondary Abutment Syndromes of the Wrist in Trauma: a Pictorial Essay
Mespreuve, M, et al. Secondary Abutment Syndromes of the Wrist in Trauma: A Pictorial Essay. Journal of the Belgian Society of Radiology. 2018; 102(1): 54, 1–8. DOI: https://doi.org/10.5334/jbsr.1558 PICTORIAL ESSAY Secondary Abutment Syndromes of the Wrist in Trauma: A Pictorial Essay Marc Mespreuve*,†, Karl Waked‡ and Koenraad Verstraete† Traumatic lesions of the wrist occur frequently and may give rise to underdiagnosed secondary abutment syndromes. The latter are a common cause of incapacitating pain and limited range of motion, despite minimal or even absent alterations on radiographs. Moreover, the complex wrist anatomy often results in ignorance or underappreciation of these syndromes. This paper presents a pictorial review of frequent and rare secondary abutment syndromes at the wrist joint, which – in contrast to primary abutment syndromes – are not based on anatomical variants or congenitaldeformations.Themeritofeachimagingmodalityisbrieflymentioned. Keywords: wrist; abutment; trauma; radiographs; MRI Introduction surfaces chondromalacia, subchondral cyst formation, and Traumatic wrist lesions occur frequently. Subsequently, surrounding synovitis. secondary abutment syndromes (SAS), a common cause of incapacitating pain and limited range of motion in spite Clinical manifestation of minimal or absent alterations on radiographs, may SAS may give rise to complaints, sometimes appearing years arise. They are often underappreciated due to the complex after trauma. The predominant symptoms are restricted wrist anatomy and call for a thorough analysis of all wrist motion and incapacitating pain, exacerbated by activity. components. SAS may have a negative impact on the three-dimensional The aim of this pictorial review is to present an overview hand positioning during daily activities [5]. -

Homologies of the Carpal Bones in Flying Squirrels (Pteromyinae): a Review
Mammal Study 26: 61-68 (2001) •. R . © the Mammalogical Society of Japan ' ,u" •XCTrc" Homologies of the carpal bones in flying squirrels (Pteromyinae): a review Richard W. Thorington, Jr.1 and Brian J. Stafford2 1 ^Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-0108 USA 2Department of Anatomy, Howard University College of Medicine, 520 W Street, N.W., Washington, DC 20059 USA Abstract. The homologies of the carpal bones of flying squirrels, presented by Oshida et al. (2000a, b), are reviewed, together with the evidence supporting traditional homology assessments. Evidence for the homology of the styliform cartilage of flying squirrels with the hypothenar cartilage of other squirrels is also reviewed. Development, articulations, topography, and muscle insertions favor both the traditional hypothesis of homology assess- ments of the carpal bones and also the hypothesis that the styliform cartilage is homologous with the hypothenar cartilage. Key words: carpal homologies, flying squirrels, Pteromyinae, styliform cartilage. In two papers, Oshida et al. (2000a, b) described the styliform cartilage of flying squirrels and suggested that it is homologous with the pisiform bone of other mammals. This is a revolutionary interpretation of the homology of the carpus. It contrasts with the hypothe- sis of Thorington et al. (1998) that the styliform cartilage of flying squirrels is homologous with the hypothenar cartilage of other squirrels. In addition, the homology assessments of Oshida et al. (2000a, b) for all the proximal carpal bones differ fundamentally from the more traditional hypothesis followed by many authors, e.g. Hill (1937), Bryant (1945), Holmgren (1952), Grasse and Dekeyser (1955), Thorington (1984), Thorington et al. -

Therapeutic Effect of Bone Cement Injection in the Treatment of Intraosseous Ganglion of the Carpal Bones
EXPERIMENTAL AND THERAPEUTIC MEDICINE 12: 1537-1541, 2016 Therapeutic effect of bone cement injection in the treatment of intraosseous ganglion of the carpal bones KUNLUN YU, XINZHONG SHAO, DEHU TIAN, JIANGBO BAI, BING ZHANG and YINGZE ZHANG Department of Orthopedic Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei 050051, P.R. China Received March 14, 2015; Accepted April 19, 2016 DOI: 10.3892/etm.2016.3487 Abstract. The aim of the present study was to treat intraos- traditional treatment (10,11). However, the anatomical struc- seous ganglia of the carpal bones with injectable bone cement ture of the wrist joint is complicated, and thus the surgical grafting. Between January 2012 and December 2013, 4 patients incision may damage this structure, leading to multiple (3 men and 1 woman) presenting with wrist pain and activity complications, including joint stiffness and disturbances of the limitation were diagnosed with intraosseous ganglion of the fragile vascular system of the lunate bone (12). carpal bones by radiography. The patients were treated with Various artificial substances have been developed and minimal invasive curettage and bone cement injection surgery. used as bone grafting materials. For instance, bone cement All patients were followed up for a mean time of 17 months is a grafting material with several advantages, including ease (range, 12-22 months). The wrist pain was significantly of handling, biological safety and sufficient compressive reduced in all patients following surgery. In addition, the strength (6). Therefore, the present study used bone cement activity range and grip strength were also improved compared injection to treat 4 patients with intraosseous ganglia of the with the preoperative parameters. -

Kienböck's Disease
Kienböck’s Disease Kienbock’s disease, also known as avascular necrosis of • Tenderness directly over the lunate bone the lunate, is a condition in which the lunate bone, one of • Decreased motion or stiffness of the wrist eight small bones in the wrist, loses its blood supply, lead- • Swelling ing to death of the bone. The lunate is a central bone in the wrist that is important for proper movement and sup- Diagnosis port of the joint (Figure 1). It works closely with the two The diagnosis of Kienbock’s disease can often be made forearm bones (radius and ulna) to help the wrist move. by reviewing your history, performing a physical exami- nation, and taking x-rays. In early stages of this disease, Damage to the lunate can lead to pain, stiffness, and the x-rays may be normal and other tests are needed to sometimes arthritis of the wrist if some time has passed. confirm the diagnosis. Most likely, the most reliable test Kienbock’s disease is most common in men between the to assess the blood supply of the lunate is Magnetic Reso- ages of 20 and 40 and rarely affects both wrists. nance Imaging (MRI). CT scanning, specialized CT scanning and bone scan may also be used. Causes There is no single cause of Kienbock’s disease. It can be This is a slow-progressing disease, and patients often caused by multiple factors such as: have the condition for months or even years before they seek treatment. It can be difficult to diagnose in its earlier • Skeletal variations: The ulna can be shorter than stages. -

Anatomy Lab 8- Upper Lower Limb Joints.Pub
Lab 8—Joints of Upper/Lower Limb Acromioclavicular Joint– QuesƟons 1 of 1 1. An 18-year-old man injures the right shoulder from a down- ward blow on the point of the shoulder. The acromion of the scapula lies anteroinferior to the lateral end of the clavicle. There is tenderness in the region between the acromion and the lateral end of the clavicle and pain when abducting the right arm up to or above the level of the shoulder. Anteroposterior (AP) radiographs show that the acromioclavicular and coracoclavicu- lar spaces in the right shoulder are each more than 50% wider than the corresponding spaces in the left shoulder. The AP radi- ographs of the shoulders indicate which of the following liga- ments is significantly ruptured in the right shoulder? A. costoclavicular ligament B. coracoacromial ligament C. coracohumeral ligament D. coracoclavicular ligament E. suprascapular ligament 2. An 18-year-old man injures the right shoulder from a down- ward blow on the point of the shoulder. The acromion of the scapula lies anteroinferior to the lateral end of the clavicle. There is tenderness in the region between the acromion and the lateral end of the clavicle and pain when abducting the right arm up to or above the level of the shoulder. Anteroposterior (AP) radiographs show that the acromioclavicular and coracoclavicu- lar spaces in the right shoulder are each more than 50% wider than the corresponding spaces in the left shoulder. Which bony structure lies DIRECTLY beneath the skin at the point of the shoulder? A. acromion of the scapula B. -

THE WRIST JOINT by R
A DETAILED STUDY OF MOVEMENT OF THE WRIST JOINT By R. DOUGLAS WRIGHT, M.S. INTRODUCTION THE most precise and the most differentiated movements of the limbs are those which occur in the hand. It is generally assumed that this refinement is based on the mobility of the thumb and the lengthening of the digits asso- ciated with a highly developed neuromuscular mechanism. On closer study there appears a much more stable basis for this free but accurate movement. The range of mobility of the wrist joint is as great as that of the shoulder joint, but added to this range of mobility there is a constant steadiness resulting from the intricate admixture of arthrodial, enarthrodial, and ginglymus types of articulation. The descriptions which are given in text-books of the actual movements of the carpus are very abbreviated, and in most cases inaccurate. Apart from the anatomical exercise of understanding the changes in the axes of the short bones taking part in the most complicated joint in the body, the movements of this joint in the use of the hand and the increasingly accurate recognition of conditions of disease and trauma in the carpal bones and associated joints render a precise knowledge of movements of these essential to accurate clinical diagnosis, treatment and prognosis. METHOD OF INVESTIGATION The kinematics of the wrist joint have been studied by Fick(l), by in- spection of the moving dead joint and plane X-ray photographs; by Bryce(2) using plane X-ray photographs of the living hand; by Johnston (3) who fixed the dead joint in varying positions and then dissected the bones, and by van Bonin (4) who investigated the movements of a living hand by stereoscopic X-ray films.