1 SUPPLEMENTARY INFORMATION Supplementary Information Related
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification of the Binding Partners for Hspb2 and Cryab Reveals
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2013-12-12 Identification of the Binding arP tners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non- Redundant Roles for Small Heat Shock Proteins Kelsey Murphey Langston Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Microbiology Commons BYU ScholarsArchive Citation Langston, Kelsey Murphey, "Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins" (2013). Theses and Dissertations. 3822. https://scholarsarchive.byu.edu/etd/3822 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Julianne H. Grose, Chair William R. McCleary Brian Poole Department of Microbiology and Molecular Biology Brigham Young University December 2013 Copyright © 2013 Kelsey Langston All Rights Reserved ABSTRACT Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactors and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston Department of Microbiology and Molecular Biology, BYU Master of Science Small Heat Shock Proteins (sHSP) are molecular chaperones that play protective roles in cell survival and have been shown to possess chaperone activity. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Genome-Wide Identification, Characterization and Expression
G C A T T A C G G C A T genes Article Genome-Wide Identification, Characterization and Expression Profiling of myosin Family Genes in Sebastes schlegelii Chaofan Jin 1, Mengya Wang 1,2, Weihao Song 1 , Xiangfu Kong 1, Fengyan Zhang 1, Quanqi Zhang 1,2,3 and Yan He 1,2,* 1 MOE Key Laboratory of Molecular Genetics and Breeding, College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China; [email protected] (C.J.); [email protected] (M.W.); [email protected] (W.S.); [email protected] (X.K.); [email protected] (F.Z.); [email protected] (Q.Z.) 2 Laboratory of Tropical Marine Germplasm Resources and Breeding Engineering, Sanya Oceanographic Institution, Ocean University of China, Sanya 572000, China 3 Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266003, China * Correspondence: [email protected]; Tel.: +86-0532-82031986 Abstract: Myosins are important eukaryotic motor proteins that bind actin and utilize the energy of ATP hydrolysis to perform a broad range of functions such as muscle contraction, cell migration, cytokinesis, and intracellular trafficking. However, the characterization and function of myosin is poorly studied in teleost fish. In this study, we identified 60 myosin family genes in a marine teleost, black rockfish (Sebastes schlegelii), and further characterized their expression patterns. myosin showed divergent expression patterns in adult tissues, indicating they are involved in different types and Citation: Jin, C.; Wang, M.; Song, W.; compositions of muscle fibers. Among 12 subfamilies, S. schlegelii myo2 subfamily was significantly Kong, X.; Zhang, F.; Zhang, Q.; He, Y. -

IDENTIFICATION and CHARACTERIZATION of ACTIN-REGULATORY PROTEINS in the HAIR CELL's CUTICULAR PLATE by LANA MARY POLLOCK Subm
IDENTIFICATION AND CHARACTERIZATION OF ACTIN-REGULATORY PROTEINS IN THE HAIR CELL’S CUTICULAR PLATE by LANA MARY POLLOCK Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy Dissertation advisor: Brian M. McDermott Jr., Ph.D. Department of Genetics and Genome Sciences CASE WESTERN RESERVE UNIVERSITY January 2016 Case Western Reserve University School of Graduate Studies We, the thesis committee, hereby approve the thesis/dissertation of Lana Pollock, candidate for the degree of Doctor of Philosophy (PhD).* (signed)_________Zhenghe Wang, Ph.D._________________ (chair of committee) ___________Brian McDermott, Ph.D._______________ ___________ Hua Lou, Ph.D._____________________ ___________Stephen Maricich, Ph.D., M.D.___________ ___________Anthony Wynshaw-Boris, Ph.D., M.D._____ Date of defense_____September 8th, 2015_______________ *we also certify that written approval has been obtained for release of any proprietary material contained therein 2 This thesis is dedicated to Daniel Margevicius. Thank you for your unwavering love and support. Ačiū!! 3 Table of contents List of Tables ........................................................................................................ 7 List of Figures ....................................................................................................... 8 List of abbreviations ............................................................................................ 13 Abstract ............................................................................................................. -

Supp Table 6.Pdf
Supplementary Table 6. Processes associated to the 2037 SCL candidate target genes ID Symbol Entrez Gene Name Process NM_178114 AMIGO2 adhesion molecule with Ig-like domain 2 adhesion NM_033474 ARVCF armadillo repeat gene deletes in velocardiofacial syndrome adhesion NM_027060 BTBD9 BTB (POZ) domain containing 9 adhesion NM_001039149 CD226 CD226 molecule adhesion NM_010581 CD47 CD47 molecule adhesion NM_023370 CDH23 cadherin-like 23 adhesion NM_207298 CERCAM cerebral endothelial cell adhesion molecule adhesion NM_021719 CLDN15 claudin 15 adhesion NM_009902 CLDN3 claudin 3 adhesion NM_008779 CNTN3 contactin 3 (plasmacytoma associated) adhesion NM_015734 COL5A1 collagen, type V, alpha 1 adhesion NM_007803 CTTN cortactin adhesion NM_009142 CX3CL1 chemokine (C-X3-C motif) ligand 1 adhesion NM_031174 DSCAM Down syndrome cell adhesion molecule adhesion NM_145158 EMILIN2 elastin microfibril interfacer 2 adhesion NM_001081286 FAT1 FAT tumor suppressor homolog 1 (Drosophila) adhesion NM_001080814 FAT3 FAT tumor suppressor homolog 3 (Drosophila) adhesion NM_153795 FERMT3 fermitin family homolog 3 (Drosophila) adhesion NM_010494 ICAM2 intercellular adhesion molecule 2 adhesion NM_023892 ICAM4 (includes EG:3386) intercellular adhesion molecule 4 (Landsteiner-Wiener blood group)adhesion NM_001001979 MEGF10 multiple EGF-like-domains 10 adhesion NM_172522 MEGF11 multiple EGF-like-domains 11 adhesion NM_010739 MUC13 mucin 13, cell surface associated adhesion NM_013610 NINJ1 ninjurin 1 adhesion NM_016718 NINJ2 ninjurin 2 adhesion NM_172932 NLGN3 neuroligin -
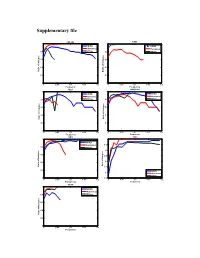
Supplementary File
Supplementary file COL1A2 FZD3 1 1 HPRD HPRD Humannet Humannet 0.8 PPIwu 0.8 PPIwu 0.6 0.6 0.4 0.4 Ratio of of indegree Ratio Ratio of Indegree 0.2 0.2 0 0 0 0.05 0.1 0.15 0.2 0 0.05 0.1 0.15 0.2 Frequency Frequency RBL1 SMARCA4 1 1 HPRD HPRD Humannet Humannet 0.8 PPIwu 0.8 PPIwu 0.6 0.6 0.4 0.4 Ratio of Indegree Ratio of Indegree Ratio 0.2 0.2 0 0 0 0.05 0.1 0.15 0.2 0 0.05 0.1 0.15 0.2 Frequency Frequency SRC TP53 1 1 HPRD Humannet 0.95 0.8 PPIwu 0.9 e 0.6 0.85 0.8 0.4 Ratio of Indegree of Ratio Ratio of Indegre 0.75 0.2 HPRD 0.7 Humannet PPIwu 0 0.65 0 0.05 0.1 0.15 0.2 0 0.05 0.1 0.15 0.2 Frequency Frequency VCAN 1 HPRD Humannet 0.8 PPIwu 0.6 0.4 Ratio of Indegree 0.2 0 0 0.05 0.1 0.15 0.2 Frequency Figure S1. Variation of indegree ratio with frequency cutoffs from 0 to 0.2 with a step 0.002 for driver genes common by three fitness cores in COAD. The indegree ratio is set to NULL if total degree of this genes is less than 5. BRAF CASR 1 1 0.8 0.8 0.6 0.6 0.4 0.4 Ratio of Indegree Ratio of Indegree of Ratio HPRD HPRD 0.2 0.2 Humannet Humannet PPIwu PPIwu 0 0 0 0.05 0.1 0.15 0.2 0 0.05 0.1 0.15 0.2 Frequency Frequency HDAC9 NRAS 1 1 0.8 0.8 0.6 0.6 0.4 0.4 HPRD HPRD Ratio of Indegree Ratio of Indegree 0.2 0.2 Humannet Humannet PPIwu PPIwu 0 0 0 0.05 0.1 0.15 0.2 0 0.05 0.1 0.15 0.2 Frequency Frequency NF1 CNTNAP2 1 1 0.9 0.8 0.8 0.6 0.7 0.4 HPRD HPRD Ratio of Indegree Ratio of Indegree 0.6 Humannet 0.2 Humannet PPIwu PPIwu 0.5 0 0 0.05 0.1 0.15 0.2 0 0.05 0.1 0.15 0.2 Frequency Frequency Figure S2. -

Myosin IIIB Uses an Actin-Binding Motif in Its Espin-1 Cargo to Reach the Tips of Actin Protrusions
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 22, 320–325, February 21, 2012 ª2012 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2011.12.053 Report Myosin IIIB Uses an Actin-Binding Motif in Its Espin-1 Cargo to Reach the Tips of Actin Protrusions Raymond C. Merritt,1,2,5 Uri Manor,1,5 Felipe T. Salles,1,5 domain (THDII; [3]), failed to localize to filopodia tips (Fig- M’hamed Grati,1 Andrea C. Dose,3 William C. Unrath,4 ure 1A). As predicted by the inchworm hypothesis that Omar A. Quintero,4 Christopher M. Yengo,4 depends on the actin-binding activity of the THDII [1], and Bechara Kachar1,* MYO3A lacking the THDII (GFP-MYO3ADTHDII) also failed to 1Laboratory of Cell Structure and Dynamics, National Institute localize to filopodia tips (Figure 1A). However, when we fused on Deafness and Other Communication Disorders, a THDII from MYO3A onto the C terminus of MYO3B (cherry- National Institutes of Health, Bethesda, MD 20892, USA MYO3B:THDII), the hybrid protein localized to the tips of filo- 2Department of Biology, Gallaudet University, Washington, podia (Figure 1A). DC 20002, USA To confirm that the THDII interacts with actin in cells, we 3Department of Molecular and Cell Biology, University of transfected COS7 cells with GFP tagged MYO3A tail California, Berkeley, Berkeley, CA 94720, USA constructs containing the THDII, and we found that the GFP 4Department of Cellular and Molecular Physiology, Penn State fluorescence colocalized with actin (Figure 1B). -

A Novel JAK1 Mutant Breast Implant-Associated Anaplastic Large Cell Lymphoma Patient-Derived Xenograft Fostering Pre- Clinical Discoveries
Cancers 2019 S1 of S18 Supplementary Materials: A Novel JAK1 Mutant Breast Implant-Associated Anaplastic Large Cell Lymphoma Patient-Derived Xenograft Fostering Pre- Clinical Discoveries Danilo Fiore, Luca Vincenzo Cappelli, Paul Zumbo, Jude M. Phillip, Zhaoqi Liu, Shuhua Cheng, Liron Yoffe, Paola Ghione, Federica Di Maggio, Ahmet Dogan, Inna Khodos, Elisa de Stanchina, Joseph Casano, Clarisse Kayembe, Wayne Tam, Doron Betel, Robin Foa’, Leandro Cerchietti, Raul Rabadan, Steven Horwitz, David M. Weinstock and Giorgio Inghirami A B C Figure S1. (A) Histology micrografts on IL89 PDTX show overall similarity between T1 T3 and T7 passages (upper panels). Immunohistochemical stains with the indicated antibodies (anti-CD3, anti- CD25 and anti-CD8 [x20]) (lower panels). (B) Flow cytometry panel comprehensive of the most represented surface T-cell lymphoma markers, including: CD2, CD3, CD4, CD5, CD8, CD16, CD25, CD30, CD56, TCRab, TCRgd. IL89 PDTX passage T3 is here depicted for illustration purposes. (C) Analysis of the TCR gamma specific rearrangement clonality in IL89 diagnostic sample and correspondent PDTX after 1 and 5 passages (T1 and T5). A WT Primary p.G1097D IL89 T1 p.G1097D IL89 T5 p.G1097D IL89 cell line B Figure S2. (A) Sanger sequencing confirms the presence of the JAK1 p.G1097D mutation in IL89 PDTX samples and in the cell line, but the mutation is undetectable in the primary due to the low sensitivity of the technique. (B) Manual backtracking of mutations in the primary tumor using deep sequencing data allowed for the identification of several hits at a very low VAF compared to the PDTX-T5. A B IL89 CTRL 30 CTRL Ruxoli?nib S 20 M Ruxoli?nib A R G 10 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 1 1 1 1 1 WEEKS AFTER ENGRAFTMENT Figure S3. -

Stereocilia-Staircase Spacing Is Influenced by Myosin III Motors And
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Special Education and Communication Department of Special Education and Disorders Faculty Publications Communication Disorders 2016 Stereocilia-staircase spacing is influenced yb myosin III motors and their cargos espin-1 and espin-like Seham Ebrahim National Institutes of Health, Bethesda, Maryland Matthew R. Avenarius Oregon Health & Science University, Portland M’hamed Grati National Institutes of Health, Bethesda, Maryland Jocelyn F. Krey Oregon Health & Science University, Portland Alanna M. Windsor National Institutes of Health, Bethesda, Maryland See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/specedfacpub Part of the Special Education and Teaching Commons Ebrahim, Seham; Avenarius, Matthew R.; Grati, M’hamed; Krey, Jocelyn F.; Windsor, Alanna M.; Sousa, Aurea D.; Ballesteros, Angela; Cui, Runjia; Millis, Bryan A.; Salles, Felipe T.; Baird, Michelle A.; Davidson, Michael W.; Jones, Sherri M.; Choi, Dongseok; Dong, Lijin; Raval, Manmeet H.; Yengo, Christopher M.; Gillespie, Peter G. Barr-; and Kachar, Bechara, "Stereocilia-staircase spacing is influenced yb myosin III motors and their cargos espin-1 and espin-like" (2016). Special Education and Communication Disorders Faculty Publications. 100. https://digitalcommons.unl.edu/specedfacpub/100 This Article is brought to you for free and open access by the Department of Special Education and Communication Disorders at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Special Education and Communication Disorders Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Seham Ebrahim, Matthew R. Avenarius, M’hamed Grati, Jocelyn F. Krey, Alanna M. Windsor, Aurea D. -

Unconventional Myosin 3A and Human
The Pennsylvania State University The Graduate School Department of Physiology A SILENT SYMPHONY: UNCONVENTIONAL MYOSIN 3A AND HUMAN HEREDITARY HEARING LOSS A Thesis in Anatomy by Lina Jamis © 2015, Lina Jamis Submitted in Partial Fulfillment of the Requirements for the degree of Master of Science August 2015 The thesis of Lina Jamis was reviewed and *approved by the following: Christopher Yengo Associate Professor of Cellular and Molecular Physiology Thesis Advisor Alistair Barber Associate Professor of Ophthalmology and Cellular & Molecular Physiology Sarah Bronson Professor and Director of Research Development and Interdisciplinary Research Patricia McLaughlin Professor of Neural and Behavioral Sciences Chair of the Graduate Program in Anatomy *Signatures are on file in the Graduate School. ii A Silent Symphony: Unconventional Myosin3A and Human Hereditary Hearing Loss Lina Jamis (ABSTRACT) The perception of sound is a process that converts mechanical sound waves to nerve impulses and relies heavily on actin-based protrusions in inner ear hair cells called stereocilia. Unconventional class III myosins (MYO3A and MYO3B) have been found to play a role in the maintenance of stereocilia length, which is essential for hearing. A number of proteins have been implicated in stereocilia length maintenance and many are associated with non-syndromic hereditary hearing loss. ESPIN1 is an actin bundling protein that is transported by MYO3 to the barbed-end of the stereocilia where it plays a role in cross-linking and regulating actin filament length. Recessive loss-of-function mutations in MYO3A are the cause of the inherited hearing impairment phenotype DFNB30 (Walsh et al., 2002), possibly because of the failed MYO3A-dependent ESPIN1 transport to the stereocilia tips. -

Supplemental Figure 1. Protein-Protein Interaction Network with Increased Expression in Fteb During the Luteal Phase
Supplemental Figure 1. Protein-protein interaction network with increased expression in FTEb during the luteal phase. Supplemental Figure 2. Protein-protein interaction network with decreased expression in FTEb during luteal phase. LEGENDS TO SUPPLEMENTAL FIGURES Supplemental Figure 1. Protein-protein interaction network with increased expression in FTEb during the luteal phase. Submission of probe sets differentially expressed in the FTEb specimens that clustered with SerCa as well as those specifically altered in FTEb luteal samples to the online I2D database revealed overlapping networks of proteins with increased expression in the four FTEb samples and/or FTEb luteal samples overall. Proteins are represented by nodes, and known and predicted first-degree interactions are represented by solid lines. Genes encoding proteins shown as large ovals highlighted in blue were exclusively found in the first comparison (Manuscript Figure 2), whereas those highlighted in red were only found in the second comparison (Manuscript Figure 3). Genes encoding proteins shown as large ovals highlighted in black were found in both comparisons. The color of each node indicates the ontology of the corresponding protein as determined by the Online Predicted Human Interaction Database (OPHID) link with the NAViGaTOR software. Supplemental Figure 2. Protein-protein interaction network with decreased expression in FTEb during the luteal phase. Submission of probe sets differentially expressed in the FTEb specimens that clustered with SerCa as well as those specifically altered in FTEb luteal samples to the online I2D database revealed overlapping networks of proteins with decreased expression in the four FTEb samples and/or FTEb luteal samples overall. Proteins are represented by nodes, and known and predicted first-degree interactions are represented by solid lines. -

Ultrarare Heterozygous Pathogenic Variants of Genes Causing Dominant Forms of Early-Onset Deafness Underlie Severe Presbycusis
Ultrarare heterozygous pathogenic variants of genes causing dominant forms of early-onset deafness underlie severe presbycusis Sophie Bouchera,b,c,d, Fabienne Wong Jun Taia, Sedigheh Delmaghania, Andrea Lellia, Amrit Singh-Estivaleta,b, Typhaine Duponta, Magali Niasme-Grarea,e, Vincent Michela, Nicolas Wolfff, Amel Bahloula, Yosra Bouyacouba, Didier Bouccarag, Bernard Fraysseh, Olivier Deguineh, Lionel Colleti, Hung Thai-Vana,j,k, Eugen Ionescuj, Jean-Louis Kemenyl, Fabrice Giraudetm,n, Jean-Pierre Lavieilleo, Arnaud Devèzeo, Anne-Laure Roudevitch-Pujolp, Christophe Vincentq, Christian Renardr, Valérie Franco-Vidals, Claire Thibult-Apts, Vincent Darrouzets, Eric Bizaguett, Arnaud Coezt,u,v, Hugues Aschardw, Nicolas Michalskia, Gaëlle M. Lefevrea, Anne Auboisp, Paul Avana,m,n,x,1, Crystel Bonneta,b,1, and Christine Petita,y,1,2 aInstitut de l’Audition, Institut Pasteur, INSERM, 75012 Paris, France; bComplexité du Vivant, Sorbonne Universités, Université Pierre et Marie Curie, Université Paris 06, 75005 Paris, France; cCentre Hospitalier Universitaire, Service d’Oto-Rhino-Laryngologie et Chirurgie Cervico-Faciale, 49100 Angers, France; dFaculté de Médecine, Unité de Formation et de Recherche Santé, Université d’Angers, 49100 Angers, France; eService de Biochimie et Biologie Moléculaire, Hôpital d’Enfants Armand-Trousseau, Assistance Publique-Hôpitaux de Paris (AP-HP), 75012 Paris, France; fUnité Récepteurs-Canaux, Institut Pasteur, 75015 Paris, France; gHôpital Beaujon, Hôpitaux Universitaires Paris Nord val-de-Seine, AP-HP, 92110 Clichy,