Ultrarare Heterozygous Pathogenic Variants of Genes Causing Dominant Forms of Early-Onset Deafness Underlie Severe Presbycusis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
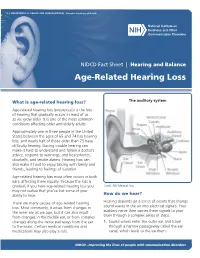
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

PSPC1 Potentiates IGF1R Expression to Augment Cell Adhesion and Motility
1 Supplementary information 2 PSPC1 potentiates IGF1R expression to augment cell 3 adhesion and motility 4 Hsin-Wei Jen1,2 , De-Leung Gu 2, Yaw-Dong Lang 2 and Yuh-Shan Jou 1,2,* 5 1 Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan 6 2 Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan 7 * Author to whom correspondence should be addressed 8 Cells 2020, 9, x; doi: FOR PEER REVIEW www.mdpi.com/journal/cells Cells 2020, 9, x FOR PEER REVIEW 2 of 10 9 10 11 Supplementary Figure S1: Expression of IGF1R and integrin in PSPC1-expressing or PSPC1-depleted 12 HCC cells by Western blotting analysis 13 (A) Detection of IGF1R protein levels in three PSPC1-knockdown cells Huh7, HepG2 and Mahlavu. (B) 14 Detection of selected integrin expression in PSPC1-overexpressing or PSPC1-depleted HCC cells by using 15 their total cell lysates immunoblotted with specific integrin antibodies as shown. 16 17 18 Supplementary Figure S2: PSPC1-modulated IGF1R downstream signaling in HCC cells. Cells 2020, 9, x FOR PEER REVIEW 3 of 10 19 (A, B) Immunoblotting of IGF1R expression in PSPC1-overexpressing SK-Hep1 and PLC5 cells 20 treated with IGF1R shRNAs. (C, D) Cell migration and adhesion were measured in PSPC1- 21 knockdown Hep3B cells rescued with exogenous expression of IGF1R. Exogenous expression of 22 IGF1R in PSPC1-knockdown Hep3B cells were then applied for detection of altered AKT/ERK 23 signaling including (E) total PSPC1, IGF1R, AKT, ERK, p-IGF1R, p-AKT(S473), and 24 p-ERK(T202/Y204) as well as altered FAK/Src signaling including (F) total FAK, Src, p-FAK(Y397) 25 and p-Src(Y416) by immunoblotting assay. -

Endoplasmic Reticulum Stress As Target for Treatment of Hearing Loss
REVIEW ARTICLE Endoplasmic reticulum stress as target for treatment of hearing loss Yanfei WANG, Zhigang XU* Shandong Provincial Key Laboratory of Animal Cell and Developmental Biology, School of Life Sciences, Shandong University, Qingdao, Shandong 266237, China *Correspondence: [email protected] https://doi.org/10.37175/stemedicine.v1i3.21 ABSTRACT The endoplasmic reticulum (ER) plays pivotal roles in coordinating protein biosynthesis and processing. Under ER stress, when excessive misfolded or unfolded proteins are accumulated in the ER, the unfolded protein response (UPR) is activated. The UPR blocks global protein synthesis while activates chaperone expression, eventually leading to the alleviation of ER stress. However, prolonged UPR induces cell death. ER stress has been associated with various types of diseases. Recently, increasing evidences suggest that ER stress and UPR are also involved in hearing loss. In the present review, we will discuss the role of ER stress in hereditary hearing loss as well as acquired hearing loss. Moreover, we will discuss the emerging ER stress-based treatment of hearing loss. Further investigations are warranted to understand the mechanisms in detail how ER stress contributes to hearing loss, which will help us develop better ER stress-related treatments. Keywords: ER stress · Unfolded protein response (UPR) · Hearing loss · Inner ear · Cochlea 1. Introduction far, which are mediated by ER stress sensors that reside The endoplasmic reticulum (ER) is a highly dynamic on the ER membranes, namely the inositol-requiring organelle in eukaryotic cells, playing important roles in enzyme 1α (IRE1α), the PKR-like ER kinase (PERK), protein synthesis, processing, folding, and transportation, and the activating transcription factor 6α (ATF6α) as well as lipid synthesis and calcium homeostasis. -

Snapshot: Formins Christian Baarlink, Dominique Brandt, and Robert Grosse University of Marburg, Marburg 35032, Germany
SnapShot: Formins Christian Baarlink, Dominique Brandt, and Robert Grosse University of Marburg, Marburg 35032, Germany Formin Regulators Localization Cellular Function Disease Association DIAPH1/DIA1 RhoA, RhoC Cell cortex, Polarized cell migration, microtubule stabilization, Autosomal-dominant nonsyndromic deafness (DFNA1), myeloproliferative (mDia1) phagocytic cup, phagocytosis, axon elongation defects, defects in T lymphocyte traffi cking and proliferation, tumor cell mitotic spindle invasion, defects in natural killer lymphocyte function DIAPH2 Cdc42 Kinetochore Stable microtubule attachment to kinetochore for Premature ovarian failure (mDia3) chromosome alignment DIAPH3 Rif, Cdc42, Filopodia, Filopodia formation, removing the nucleus from Increased chromosomal deletion of gene locus in metastatic tumors (mDia2) Rac, RhoB, endosomes erythroblast, endosome motility, microtubule DIP* stabilization FMNL1 (FRLα) Cdc42 Cell cortex, Phagocytosis, T cell polarity Overexpression is linked to leukemia and non-Hodgkin lymphoma microtubule- organizing center FMNL2/FRL3/ RhoC ND Cell motility Upregulated in metastatic colorectal cancer, chromosomal deletion is FHOD2 associated with mental retardation FMNL3/FRL2 Constituently Stress fi bers ND ND active DAAM1 Dishevelled Cell cortex Planar cell polarity ND DAAM2 ND ND ND Overexpressed in schizophrenia patients Human (Mouse) FHOD1 ROCK Stress fi bers Cell motility FHOD3 ND Nestin, sarcomere Organizing sarcomeres in striated muscle cells Single-nucleotide polymorphisms associated with type 1 diabetes -

Mutations in the WFS1 Gene Are a Frequent Cause of Autosomal Dominant Nonsyndromic Low-Frequency Hearing Loss in Japanese
J Hum Genet (2007) 52:510–515 DOI 10.1007/s10038-007-0144-3 ORIGINAL ARTICLE Mutations in the WFS1 gene are a frequent cause of autosomal dominant nonsyndromic low-frequency hearing loss in Japanese Hisakuni Fukuoka Æ Yukihiko Kanda Æ Shuji Ohta Æ Shin-ichi Usami Received: 14 January 2007 / Accepted: 27 March 2007 / Published online: 11 May 2007 Ó The Japan Society of Human Genetics and Springer 2007 Abstract Mutations in WFS1 are reported to be respon- sites are likely to be mutational hot spots. All three families sible for two conditions with distinct phenotypes; DFNA6/ with WFS1 mutations in this study showed a similar phe- 14/38 and autosomal recessive Wolfram syndrome. They notype, LFSNHL, as in previous reports. In this study, one- differ in their associated symptoms and inheritance mode, third (three out of nine) autosomal dominant LFSNHL and although their most common clinical symptom is families had mutations in the WFS1 gene, indicating that in hearing loss, it is of different types. While DNFA6/14/38 is non-syndromic hearing loss WFS1 is restrictively and characterized by low frequency sensorineural hearing loss commonly found within autosomal dominant LFSNHL (LFSNHL), in contrast, Wolfram syndrome is associated families. with various hearing severities ranging from normal to profound hearing loss that is dissimilar to LFSNHL (Pen- Keywords WSF1 Á Low-frequency hearing loss Á nings et al. 2002). To confirm whether within non-syn- DFNA6/14/38 dromic hearing loss patients WFS1 mutations are found restrictively in patients with LFSNHL and to summarize the mutation spectrum of WFS1 found in Japanese, we Introduction screened 206 Japanese autosomal dominant and 64 auto- somal recessive (sporadic) non-syndromic hearing loss WFS1 is a gene encoding an 890 amino-acid glycoprotein probands with various severities of hearing loss. -

Fumihiko Urano: Wolfram Syndrome: Diagnosis, Management, And
Curr Diab Rep (2016) 16:6 DOI 10.1007/s11892-015-0702-6 OTHER FORMS OF DIABETES (JJ NOLAN, SECTION EDITOR) Wolfram Syndrome: Diagnosis, Management, and Treatment Fumihiko Urano1,2 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Wolfram syndrome is a rare genetic disorder char- diabetes insipidus, optic nerve atrophy, hearing loss, and acterized by juvenile-onset diabetes mellitus, diabetes neurodegeneration. It was first reported in 1938 by Wol- insipidus, optic nerve atrophy, hearing loss, and neurodegen- fram and Wagener who found four of eight siblings with eration. Although there are currently no effective treatments juvenile diabetes mellitus and optic nerve atrophy [1]. that can delay or reverse the progression of Wolfram syn- Wolfram syndrome is considered a rare disease and esti- drome, the use of careful clinical monitoring and supportive mated to afflict about 1 in 160,000–770,000 [2, 3]. In care can help relieve the suffering of patients and improve 1995, Barrett, Bundey, and Macleod described detailed their quality of life. The prognosis of this syndrome is current- clinical features of 45 patients with Wolfram syndrome ly poor, and many patients die prematurely with severe neu- and determined the best available diagnostic criteria for rological disabilities, raising the urgency for developing novel the disease [3]. According to the draft International Clas- treatments for Wolfram syndrome. In this article, we describe sification of Diseases (ICD-11), Wolfram Syndrome is natural history and etiology, provide recommendations for categorized as a rare specified diabetes mellitus (subcate- diagnosis and clinical management, and introduce new treat- gory 5A16.1, Wolfram Syndrome). -

Identification of the Binding Partners for Hspb2 and Cryab Reveals
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2013-12-12 Identification of the Binding arP tners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non- Redundant Roles for Small Heat Shock Proteins Kelsey Murphey Langston Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Microbiology Commons BYU ScholarsArchive Citation Langston, Kelsey Murphey, "Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins" (2013). Theses and Dissertations. 3822. https://scholarsarchive.byu.edu/etd/3822 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactions and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Julianne H. Grose, Chair William R. McCleary Brian Poole Department of Microbiology and Molecular Biology Brigham Young University December 2013 Copyright © 2013 Kelsey Langston All Rights Reserved ABSTRACT Identification of the Binding Partners for HspB2 and CryAB Reveals Myofibril and Mitochondrial Protein Interactors and Non-Redundant Roles for Small Heat Shock Proteins Kelsey Langston Department of Microbiology and Molecular Biology, BYU Master of Science Small Heat Shock Proteins (sHSP) are molecular chaperones that play protective roles in cell survival and have been shown to possess chaperone activity. -

Myosin Myth4-FERM Structures Highlight Important Principles of Convergent Evolution
Myosin MyTH4-FERM structures highlight important principles of convergent evolution Vicente José Planelles-Herreroa,b, Florian Blanca,c, Serena Sirigua, Helena Sirkiaa, Jeffrey Clausea, Yannick Souriguesa, Daniel O. Johnsrudd, Beatrice Amiguesa, Marco Cecchinic, Susan P. Gilberte, Anne Houdussea,1,2, and Margaret A. Titusd,1,2 aStructural Motility, Institut Curie, CNRS, UMR 144, PSL Research University, F-75005 Paris, France; bUPMC Université de Paris 6, Institut de Formation Doctorale, Sorbonne Universités, 75252 Paris Cedex 05, France; cLaboratoire d’Ingénierie des Fonctions Moléculaires, Institut de Science et d’Ingénierie Supramoléculaires, UMR 7006 CNRS, Université de Strasbourg, F-67083 Strasbourg Cedex, France; dDepartment of Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN 55455; and eDepartment of Biological Sciences, Rensselaer Polytechnic Institute, Troy, NY 12180 Edited by James A. Spudich, Stanford University School of Medicine, Stanford, CA, and approved March 31, 2016 (received for review January 15, 2016) Myosins containing MyTH4-FERM (myosin tail homology 4-band (Fig. 1). These MF myosins are widespread and likely quite an- 4.1, ezrin, radixin, moesin, or MF) domains in their tails are found cient because they are found in many different branches of the in a wide range of phylogenetically divergent organisms, such as phylogenetic tree (5, 6), including Opisthokonts (which includes humans and the social amoeba Dictyostelium (Dd). Interestingly, Metazoa, unicellular Holozoa, and Fungi), Amoebozoa, and the evolutionarily distant MF myosins have similar roles in the exten- SAR (Stramenopiles, Alveolates, and Rhizaria) (Fig. 1 A and B). sion of actin-filled membrane protrusions such as filopodia and Over the course of hundreds of millions years of parallel evolution bind to microtubules (MT), suggesting that the core functions of the MF myosins have acquired or maintained roles in the formation these MF myosins have been highly conserved over evolution. -

Report Mutations in a Novel Isoform of TRIOBP That Encodes A
Report Mutations in a Novel Isoform of TRIOBP That Encodes a Filamentous- Actin Binding Protein Are Responsible for DFNB28 Recessive Nonsyndromic Hearing Loss Hashem Shahin,1,2 Tom Walsh,3 Tama Sobe,2 Judeh Abu Sa’ed,1 Amal Abu Rayan,1 Eric D. Lynch,3 Ming K. Lee,3 Karen B. Avraham,2 Mary-Claire King,3 and Moein Kanaan1 1Department of Life Sciences, Bethlehem University, Bethlehem; 2Department of Human Molecular Genetics and Biochemistry, Sackler School of Medicine, Tel Aviv University, Tel Aviv; and 3Departments of Medicine and Genome Sciences, University of Washington, Seattle In a large consanguineous Palestinian kindred, we previously mapped DFNB28—a locus associated with recessively inherited, prelingual, profound sensorineural hearing impairment—to chromosome 22q13.1. We report here that mutations in a novel 218-kDa isoform of TRIOBP (TRIO and filamentous actin [F-actin] binding protein) are associated with DFNB28 hearing loss in a total of nine Palestinian families. Two nonsense mutations (R347X and Q581X) truncate the protein, and a potentially deleterious missense mutation (G1019R) occurs in a conserved motif in a putative SH3-binding domain. In seven families, 27 deaf individuals are homozygous for one of the nonsense mutations; in two other families, 3 deaf individuals are compound heterozygous for the two nonsense mutations or for Q581X and G1019R. The novel long isoform of TRIOBP has a restricted expression profile, including cochlea, retina, and fetal brain, whereas the original short isoform is widely expressed. Antibodies to TRIOBP reveal expression in sensory cells of the inner ear and colocalization with F-actin along the length of the stereocilia. -

Novel Association of Hypertrophic Cardiomyopathy, Sensorineural Deafness, and a Mutation in Unconventional Myosin VI (MYO6)
309 LETTER TO JMG J Med Genet: first published as 10.1136/jmg.2003.011973 on 1 April 2004. Downloaded from Novel association of hypertrophic cardiomyopathy, sensorineural deafness, and a mutation in unconventional myosin VI (MYO6) S A Mohiddin, Z M Ahmed, A J Griffith, D Tripodi, T B Friedman, L Fananapazir, R J Morell ............................................................................................................................... J Med Genet 2004;41:309–314. doi: 10.1136/jmg.2003.011973 amilial hypertrophic cardiomyopathy (FHC) is typically Key points characterised by left ventricular hypertrophy, diastolic Fdysfunction, and hypercontractility, and is often asso- ciated with disabling symptoms, arrhythmias, and sudden N Familial hypertrophic cardiomyopathy (FHC) is typi- death.1 FHC shows both non-allelic and allelic genetic cally confined to a cardiac phenotype and is caused by heterogeneity, and results from any one of more than 100 mutations in genes encoding sarcomeric proteins. mutations in genes encoding sarcomeric proteins.2 Identified Occasionally FHC may be one component of a genes include those encoding b myosin heavy chain, the hereditary multisystem disorder. myosin regulatory and essential light chains, myosin bind- N Sensorineural hearing loss is genetically heteroge- ing protein C, troponin I, troponin C, a cardiac actin, and neous. Mutations in the MYO6 gene, encoding 23 titin. The FHC phenotype is characterised by hypertrophy, unconventional myosin VI, have been found to cause myocyte disarray and fibrosis, and results from the dominant non-syndromic sensorineural hearing loss—that is, negative expression of one of these (mainly missense) sensorineural hearing loss in the absence of any other mutations. The resulting sarcomeric dysfunction leads related clinical features. ultimately, through mechanisms that remain obscure, to pathological left ventricular remodelling. -

Notch-Wnt-Bmp Crosstalk Regulates Radial Patterning in the Mouse Cochlea in a Spatiotemporal Manner Vidhya Munnamalai1,2 and Donna M
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 4003-4015 doi:10.1242/dev.139469 RESEARCH ARTICLE Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner Vidhya Munnamalai1,2 and Donna M. Fekete1,2,3,* ABSTRACT development progresses (Munnamalai and Fekete, 2013). Wnt- The sensory cells of the mammalian organ of Corti assume a precise mediated regulation of cell proliferation is well known in many mosaic arrangement during embryonic development. Manipulation of organ systems, including the cochlea (Jacques et al., 2012). Whether Wnt signaling can modulate the proliferation of cochlear progenitors, the canonical Wnt signaling pathway intersects with the Notch, but whether Wnts are responsible for patterning compartments, or Bmp or Fgf pathways to regulate cochlear patterning remains specific hair cells within them, is unclear. To address how the precise relatively unexplored. timing of Wnt signaling impacts patterning across the radial axis, The Wnt and Notch pathways are known to crosstalk, a finding ‘ ’ mouse cochlear cultures were initiated at embryonic day 12.5 and that coined the term Wntch signaling. This interaction is context subjected to pharmacological treatments at different stages. Early dependent and can be bi-directional (Collu et al., 2014; Zak et al., changes in major patterning genes were assessed to understand the 2015). In the cochlea, Notch has a dual role in regulating lateral mechanisms underlying alterations of compartments. Results show induction early (to induce prosensory fate) and lateral inhibition that Wnt activation can promote medial cell fates by regulating later (to block HC fate) (Kiernan, 2013). Studies have shown that medially expressed Notch genes in a spatiotemporal manner. -

Defining Functional Interactions During Biogenesis of Epithelial Junctions
ARTICLE Received 11 Dec 2015 | Accepted 13 Oct 2016 | Published 6 Dec 2016 | Updated 5 Jan 2017 DOI: 10.1038/ncomms13542 OPEN Defining functional interactions during biogenesis of epithelial junctions J.C. Erasmus1,*, S. Bruche1,*,w, L. Pizarro1,2,*, N. Maimari1,3,*, T. Poggioli1,w, C. Tomlinson4,J.Lees5, I. Zalivina1,w, A. Wheeler1,w, A. Alberts6, A. Russo2 & V.M.M. Braga1 In spite of extensive recent progress, a comprehensive understanding of how actin cytoskeleton remodelling supports stable junctions remains to be established. Here we design a platform that integrates actin functions with optimized phenotypic clustering and identify new cytoskeletal proteins, their functional hierarchy and pathways that modulate E-cadherin adhesion. Depletion of EEF1A, an actin bundling protein, increases E-cadherin levels at junctions without a corresponding reinforcement of cell–cell contacts. This unexpected result reflects a more dynamic and mobile junctional actin in EEF1A-depleted cells. A partner for EEF1A in cadherin contact maintenance is the formin DIAPH2, which interacts with EEF1A. In contrast, depletion of either the endocytic regulator TRIP10 or the Rho GTPase activator VAV2 reduces E-cadherin levels at junctions. TRIP10 binds to and requires VAV2 function for its junctional localization. Overall, we present new conceptual insights on junction stabilization, which integrate known and novel pathways with impact for epithelial morphogenesis, homeostasis and diseases. 1 National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. 2 Computing Department, Imperial College London, London SW7 2AZ, UK. 3 Bioengineering Department, Faculty of Engineering, Imperial College London, London SW7 2AZ, UK. 4 Department of Surgery & Cancer, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK.