Polarography UNIT V
Polarography
Principle
1.
The simple principle of polarography is the study of solutions or of electrode processes by means of electrolysis with two electrodes, one polarizable and one unpolarizable, the former formed by mercury regularly dropping from a capillary tube. Polarography is a specific type of measurement that falls into the general category of linear-sweep voltammetry where the electrode potential is altered in a linear fashion from the initial potential to the final potential. As a linear sweep method controlled by convection/diffusion mass transport, the current vs. potential response of a polarographic experiment has the typical sigmoidal shape. What makes polarography different from other linear sweep voltammetry measurements is that polarography makes use of the dropping mercury electrode (DME) or the static mercury drop electrode.
Ilkovic Equation
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
id = k n D1/3m2/3t1/6c
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
A Typical Polarogram
Polarography UNIT V
2.
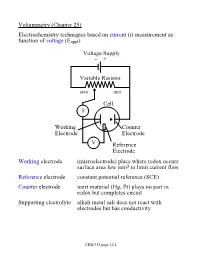
Construction and working of Dropping Mercury Electrode Construction :
The apparatus is made of three pieces 1. The Capillary 2. The Mercury reservoir vessel; and 3. The stand tube with adjoining stopcock In the capillary tube a 5.5 cm of corning marine barometer tubing is joined to 6 mm soft glass tubing. Electrical contact to the mercury in the mercury reservoir vessel is made by means of tungsten contact mercury well.
Working :
The operation of the apparatus is simple. By means of the pressure mercury can be raised to any desired height in the stand tube. The air forced in enters the reservoir vessel through a hole. As a result of the pressure, which is built up in the reservoir vessel the mercury proceeds up into a tube provided in the reservoir vessel apart from the hole from which air is forced in. The stopcock is closed when the mercury has reached the desired level. When the polarographic analysis is completed, the capillary is washed carefully with a stream of distilled water and immersed in either distilled water or mercury. Then the mercury column is lowered by opening the stopcock and the valve, whereupon the pressure in the vessel is returned to the atmospheric pressure.
Polarography UNIT V
3.
Construction and working of rotating platinum electrode Construction :
The electrode includes a conductive disk embedded in an inert non-conductive polymer or resin that can be attached to an electric motor that has very fine control of the electrode's rotation rate. The disk, like any working electrode, is generally made of a noble metal (in this case its made of Platinum) or glassy carbon, however any conductive material can be used based on specific needs.
Working :
The disk's rotation is usually described in terms of angular velocity. As the disk turns, some of the solution described as the hydrodynamic boundary layer is dragged by the spinning disk and the resulting centrifugal force flings the solution away from the centre of the electrode. Solution flows up, perpendicular to the electrode, from the bulk to replace the boundary layer. The sum result is a laminar flow of solution towards and across the electrode. The rate of the solution flow can be controlled by the electrode's angular velocity and modelled mathematically. This flow can quickly achieve conditions in which the steady-state current is controlled by the solution flow rather than diffusion. This is a contrast to still and unstirred experiments such as cyclic voltammetry where the steady-state current is limited by the diffusion of species in solution.
Polarography UNIT V
4.
PHARMACEUTICALAPPLICATIONS
(1) To identify and quantify Dissolved oxygen and peroxides. (2) For determination of Trace metals and metal-containing drugs. (3) To identify and quantify Antiseptics and insecticides. (4) To identify and quantify Vitamins. (5) To identify and quantifyHormones. (6) To identify and quantify Antibiotics. (7) Identification and quantifying of Alkaloids. (8) To identify Miscellaneous pharmaceutical substances. (9) Blood serum and cancer diagnosis.