Cyclic Voltammetry of Dopamine: an Ec Mechanism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
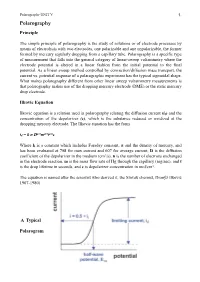
Polarography.Pdf
Polarography UNIT V !1. Polarography Principle The simple principle of polarography is the study of solutions or of electrode processes by means of electrolysis with two electrodes, one polarizable and one unpolarizable, the former formed by mercury regularly dropping from a capillary tube. Polarography is a specific type of measurement that falls into the general category of linear-sweep voltammetry where the electrode potential is altered in a linear fashion from the initial potential to the final potential. As a linear sweep method controlled by convection/diffusion mass transport, the current vs. potential response of a polarographic experiment has the typical sigmoidal shape. What makes polarography different from other linear sweep voltammetry measurements is that polarography makes use of the dropping mercury electrode (DME) or the static mercury drop electrode. Ilkovic Equation Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form id = k n D1/3m2/3t1/6c Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3. The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980). -

07 Chapter2.Pdf
22 METHODOLOGY 2.1 INTRODUCTION TO ELECTROCHEMICAL TECHNIQUES Electrochemical techniques of analysis involve the measurement of voltage or current. Such methods are concerned with the interplay between solution/electrode interfaces. The methods involve the changes of current, potential and charge as a function of chemical reactions. One or more of the four parameters i.e. potential, current, charge and time can be measured in these techniques and by plotting the graphs of these different parameters in various ways, one can get the desired information. Sensitivity, short analysis time, wide range of temperature, simplicity, use of many solvents are some of the advantages of these methods over the others which makes them useful in kinetic and thermodynamic studies1-3. In general, three electrodes viz., working electrode, the reference electrode, and the counter or auxiliary electrode are used for the measurement in electrochemical techniques. Depending on the combinations of parameters and types of electrodes there are various electrochemical techniques. These include potentiometry, polarography, voltammetry, cyclic voltammetry, chronopotentiometry, linear sweep techniques, amperometry, pulsed techniques etc. These techniques are mainly classified into static and dynamic methods. Static methods are those in which no current passes through the electrode-solution interface and the concentration of analyte species remains constant as in potentiometry. In dynamic methods, a current flows across the electrode-solution interface and the concentration of species changes such as in voltammetry and coulometry4. 2.2 VOLTAMMETRY The field of voltammetry was developed from polarography, which was invented by the Czechoslovakian Chemist Jaroslav Heyrovsky in the early 1920s5. Voltammetry is an electrochemical technique of analysis which includes the measurement of current as a function of applied potential under the conditions that promote polarization of working electrode6. -

Convergent Paired Electrochemical Synthesis of New Aminonaphthol Derivatives
www.nature.com/scientificreports OPEN New insights into the electrochemical behavior of acid orange 7: Convergent paired Received: 24 August 2016 Accepted: 29 December 2016 electrochemical synthesis of new Published: 06 February 2017 aminonaphthol derivatives Shima Momeni & Davood Nematollahi Electrochemical behavior of acid orange 7 has been exhaustively studied in aqueous solutions with different pH values, using cyclic voltammetry and constant current coulometry. This study has provided new insights into the mechanistic details, pH dependence and intermediate structure of both electrochemical oxidation and reduction of acid orange 7. Surprisingly, the results indicate that a same redox couple (1-iminonaphthalen-2(1H)-one/1-aminonaphthalen-2-ol) is formed from both oxidation and reduction of acid orange 7. Also, an additional purpose of this work is electrochemical synthesis of three new derivatives of 1-amino-4-(phenylsulfonyl)naphthalen-2-ol (3a–3c) under constant current electrolysis via electrochemical oxidation (and reduction) of acid orange 7 in the presence of arylsulfinic acids as nucleophiles. The results indicate that the electrogenerated 1-iminonaphthalen-2(1 H)-one participates in Michael addition reaction with arylsulfinic acids to form the 1-amino-3-(phenylsulfonyl) naphthalen-2-ol derivatives. The synthesis was carried out in an undivided cell equipped with carbon rods as an anode and cathode. 2-Naphthol orange (acid orange 7), C16H11N2NaO4S, is a mono-azo water-soluble dye that extensively used for dyeing paper, leather and textiles1,2. The structure of acid orange 7 involves a hydroxyl group in the ortho-position to the azo group. This resulted an azo-hydrazone tautomerism, and the formation of two tautomers, which each show an acid− base equilibrium3–12. -

Chapter 3: Experimental
Chapter 3: Experimental CHAPTER 3: EXPERIMENTAL 3.1 Basic concepts of the experimental techniques In this part of the chapter, a short overview of some phrases and theoretical aspects of the experimental techniques used in this work are given. Cyclic voltammetry (CV) is the most common technique to obtain preliminary information about an electrochemical process. It is sensitive to the mechanism of deposition and therefore provides informations on structural transitions, as well as interactions between the surface and the adlayer. Chronoamperometry is very powerful method for the quantitative analysis of a nucleation process. The scanning tunneling microscopy (STM) is based on the exponential dependence of the tunneling current, flowing from one electrode onto another one, depending on the distance between electrodes. Combination of the STM with an electrochemical cell allows in-situ study of metal electrochemical phase formation. XPS is also a very powerfull technique to investigate the chemical states of adsorbates. Theoretical background of these techniques will be given in the following pages. At an electrode surface, two fundamental electrochemical processes can be distinguished: 3.1.1 Capacitive process Capacitive processes are caused by the (dis-)charge of the electrode surface as a result of a potential variation, or by an adsorption process. Capacitive current, also called "non-faradaic" or "double-layer" current, does not involve any chemical reactions (charge transfer), it only causes accumulation (or removal) of electrical charges on the electrode and in the electrolyte solution near the electrode. There is always some capacitive current flowing when the potential of an electrode is changing. In contrast to faradaic current, capacitive current can also flow at constant 28 Chapter 3: Experimental potential if the capacitance of the electrode is changing for some reason, e.g., change of electrode area, adsorption or temperature. -

Unit 1 Introduction to Electro- Analytical Methods
Introduction to UNIT 1 INTRODUCTION TO ELECTRO- Electroanalytical ANALYTICAL METHODS Methods Structure 1.1 Introduction Objectives 1.2 Basic Concepts Electrical Units Basic Laws of Electrochemistry Electrode Potential Liquid-Junction Potentials Electrochemical Cells The Nernst Equation Cell Potential 1.3 Classification and an Overview of Electroanalytical Methods Potentiometry Voltammetry Polarography Amperometry Electrogravimetry and Coulometry Conductometry 1.4 Classification and Relationships of Electroanalytical Methods 1.5 Summary 1.6 Terminal Questions 1.7 Answers 1.1 INTRODUCTION This is the first unit of this course. This unit deals with the fundamentals of electrochemistry that are necessary for understanding the principles of electroanalytical methods discussed in this Unit 2 to 9. In this unit we have also classified of electroanalytical methods and briefly introduced of some important electroanalytical methods. More details of these elecroanalytical methods will be discussed in the consecutive units. Objectives After studying this unit, you will be able to: • name the different units of electrical quantities, • define the two basic laws of electrochemistry, • describe the single electrode potential and the potential of a galvanic cell, • derive the Nernst expression and give its applications, • calculate the electrode potentials and cell potentials using Nernst equation, • describe the basis for classification of the electroanalytical techniques, and • explain the basis principles and describe the essential conditions of the various electroanalytical techniques. 1.2 BASIC CONCEPTS Before going in detail of different electroanalytical techniques, let’s recapitulate some basic concepts which you have studied in your undergraduate classes. 7 Electroanalytical 1.2.1 Electrical Units Methods -I Ampere (A): Ampere is the unit of current. -

Stationary Electrode Voltammetry and Chronoamperometry in an Alkali Metal Carbonate-Borate Melt
AN ABSTRACT OF THE THESIS OF DARRELL GEORGE PETCOFF for the Doctor of Philosophy (Name of student) (Degree) in Analytical Chemistry presented onC (O,/97 (Major) (Date) Title: STATIONARY ELECTRODE VOLTAMMETRY AND CHRONOAMPEROMETRY IN AN ALKALI METAL CARBONATE - BORATE. MFT T Abstract approved: Redacted for Privacy- Drir. reund The electrochemistry of the lithium-potassium-sodium carbonate-borate melt was explored by voltammetry and chrono- amperometry. In support of this, a controlled-potential polarograph and associated hardware was constructed.Several different types of reference electrodes were tried before choosing a porcelain mem- brane electrode containing a silver wire immersed in a silver sulfate melt.The special porcelain compounded was used also to construct a planar gold disk electrode.The theory of stationary electrode polarography was summarized and denormalized to provide an over- all view. A new approach to the theory of the cyclic background current was also advanced. A computer program was written to facilitate data processing.In addition to providing peak potentials, currents, and n-values, the program also resolves overlapping peaks and furnishes plots of both processed and unprocessed data. Rapid-scan voltammetry was employed to explore the electro- chemical behavior of Zn, Co, Fe, Tl, Sb, As, Ni, Sn, Cd, Te, Bi, Cr, Pb, Cu, and U in the carbonate-borate melt. Most substances gave reasonably well-defined peaks with characteristic peak potentials and n-values.Metal deposition was commonly accompanied by adsorp- tion prepeaks indicative of strong adsorption, and there was also evi- dence of a preceding chemical reaction for several elements, sug- gesting decomplexation before reduction. -

Thesis-1961-B586i.Pdf
INVESTIGATION OF SOME POSSIBILITIES FOR AMPEROMETRIC TITRATION OF CERTAIN METAL IONS WITH OXINE By Donald George Biechler I I Bachelor of Science University of Wisconsin Madison, Wisconsin 1956 Submitted to the faculty of the Graduate School of the Oklahoma State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May, 1961 INVESTIGATION OF SOME POSSIBILITIES FOR AMPEROMEI'RIC TITRATION OF CERTAIN MEI'AL IONS WITH OXINE Thesis Approved: Thesis Adviser i i OKLAHOMA STATE UNIVERSITY llBRARY JAN 2 1962 PREFACE Oxine (8-hydroxyquinoline) is most generally used in analytical chemistry as a precipitant for metals and is known to form water- insoluble chelates with better than thirty metal ions (3). There exists in solutions of oxine a tautomeric equilibrium of the fol- lowing type: C C C C /~/'\ ,c/""/~ C C f ij 1 I II I C C C C C C ~/"'/C N ~/C "+/N r . _I I 0 H 0--------H Chelation of a metal ion involves replacement of the proton and for- mation of a coordinate bond with the nitrogen to form a stable 5 membered ring compound. Thus nickel, a bivalent cation, would form a compound with the following structure: 4810 90 iii iv The oxinates can be ignited and weighed as such or they may be further ignited to the metal oxides and then weighed. Alternately the oxinates may be dissolved in acid and quantitatively brominated (7)0 Considering the number of metal ions that are precipitated by oxine, it seemed that possibly more use could be made of the reagent in volumetric analysis. -

Cyclic Voltammetry
Cyclic Voltammetry Denis Andrienko January 22, 2008 2 Literature: 1. Allen J. Bard, Larry R. Faulkner “Electrochemical Methods: Fundamentals and Applications” 2. http://www.cheng.cam.ac.uk/research/groups/electrochem/teaching.html Chapter 1 Cyclic Voltammetry 1.1 Background Cyclic voltammetry is the most widely used technique for acquiring qualitative information about elec- trochemical reactions. it offers a rapid location of redox potentials of the electroactive species. A few concepts has to be introduced before talking about this method. 1.1.1 Electronegativity Electronegativity is the affinity for electrons. The atoms of the various elements differ in their affinity for electrons. The term was first proposed by Linus Pauling in 1932 as a development of valence bond theory. The table for all elements can be looked up on Wikipedia: http://en.wikipedia.org/wiki/Electronegativity. Some facts to remember: • Fluorine (F) is the most electronegative element. χF = 3.98. • The electronegativity of oxygen (O) χO = 3.44 is exploited by life, via shuttling of electrons between carbon (C, χF = 2.55) and oxygen (O): Moving electrons against the gradient (O to C) - as occurs in photosynthesis - requires energy (and stores it). Moving electrons down the gradient (C to O) - as occurs in cellular respiration - releases energy. • The relative electronegativity of two interacting atoms plays a major part in determining what kind of chemical bond forms between them. Examples: • Sodium (χNa = 0.93) and Chlorine (χCl = 3.16) = Ionic Bond: There is a large difference in electronegativity, so the chlorine atom takes an electron from the sodium atom converting the atoms into ions (Na+) and (Cl−). -

Supporting Information Redox-Active and Semi-Conducting Donor
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is © The Royal Society of Chemistry 2018 Supporting Information Redox-active and Semi-conducting Donor-Acceptor Conjugated Microporous Polymers as Metal-free ORR Catalysts Syamantak Roy,a Arkamita Bandyopadhyay,b Mrinmay Das,c Partha Pratim Ray,c Swapan K. Patib and Tapas Kumar Maji*a a Molecular Materials Laboratory, Chemistry and Physics of Materials Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore-560064, India b New Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore- 560064 c Department of Physics, Jadavpur University, Kolkata -700032 Table of Contents Physical Measurements ..................................................................................................................2 Computational Details ....................................................................................................................3 Electrochemical Measurements ......................................................................................................3 Experimental Section......................................................................................................................5 IR Spectra .......................................................................................................................................7 PXRD .............................................................................................................................................7 -
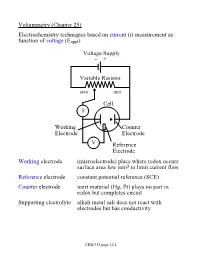
Voltammetry (Chapter 25) Electrochemistry Techniques Based on Current (I) Measurement As Function of Voltage (Eappl)
Voltammetry (Chapter 25) Electrochemistry techniques based on current (i) measurement as function of voltage (Eappl) Voltage Supply - + Variable Resistor max min Cell I Working Counter Electrode Electrode V Reference Electrode Working electrode (microelectrode) place where redox occurs surface area few mm2 to limit current flow Reference electrode constant potential reference (SCE) Counter electrode inert material (Hg, Pt) plays no part in redox but completes circuit Supporting electrolyte alkali metal salt does not react with electrodes but has conductivity CEM 333 page 12.1 Why not use 2 electrodes? OK in potentiometry - very small currents. Now, want to measure current (larger=better) but • potential drops when current is taken from electrode (IR drop) • must minimize current withdrawn from reference electrode surface Potentiostat (voltage source) drives cell • supplies whatever voltage needed between working and counter electrodes to maintain specific voltage between working and reference electrode NOTE: • Almost all current carried between working and counter electrodes • Voltage measured between working and reference electrodes • Analyte dissolved in cell not at electrode surface! CEM 333 page 12.2 Excitation signals (Fig 25-2) CEM 333 page 12.3 Microelectrodes C, Au, Pt, Hg each useful in certain solutions/voltage ranges Fig 25-4 At -ve limit, oxidation of water + - 2H2O ® 4H + O2(g) + 4e At +ve limit, reduction of water - - 2H2O + 2e ® H2 + 2OH CEM 333 page 12.4 Varies with material/solution due to different overpotentials Overpotential -

Walljet Electrochemistry: Quantifying Molecular Transport Through Metallopolymeric and Zirconium Phosphonate Assembled Porphyrin Square Thin Films
4422 Langmuir 2004, 20, 4422-4429 Walljet Electrochemistry: Quantifying Molecular Transport through Metallopolymeric and Zirconium Phosphonate Assembled Porphyrin Square Thin Films Aaron M. Massari, Richard W. Gurney, Craig P. Schwartz, SonBinh T. Nguyen, and Joseph T. Hupp* Department of Chemistry and the Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 Received January 12, 2004. In Final Form: March 7, 2004 By employing redox-active probes, condensed-phase molecular transport through nanoporous thin films can often be measured electrochemically. Certain kinds of electrode materials (e.g. conductive glass) are difficult to fabricate as rotatable disks or as ultramicroelectrodessthe configurations most often used for electrochemical permeation measurements. These limitations point to the need for a more materials- general measurement method. Herein, we report the application of walljet electrochemistry to the study of molecular transport through model metallopolymeric films on indium tin oxide electrodes. A quantitative expression is presented that describes the transport-limited current at the walljet electrode in terms of mass transport through solution and permeation through the film phase. A comparison of the film permeabilities for a series of redox probes measured using the walljet electrode and a rotating disk electrode establishes the accuracy of the walljet method, while also demonstrating similar precision for the two methods. We apply this technique to a system consisting of zirconium phosphonate assembled films of a porphyrinic molecular square. Transport through films comprising three or more layers is free from significant contributions from pinhole defects. Surprisingly, transport through films of this kind is 2-3 orders of magnitude slower than through films constructed via interfacial polymerization of nearly identical supramolecular square building blocks (Keefe; et al. -
Electroanalytical Methods: Guide to Experiments and Applications, 2Nd
Electroanalytical Methods Fritz Scholz Editor Electroanalytical Methods Guide to Experiments and Applications Second, Revised and Extended Edition With contributions by A.M. Bond, R.G. Compton, D.A. Fiedler, G. Inzelt, H. Kahlert, Š. Komorsky-Lovric,´ H. Lohse, M. Lovric,´ F. Marken, A. Neudeck, U. Retter, F. Scholz, Z. Stojek 123 Editor Prof. Dr. Fritz Scholz University of Greifswald Inst. of Biochemistry Felix-Hausdorff-Str. 4 17487 Greifswald Germany [email protected] ISBN 978-3-642-02914-1 e-ISBN 978-3-642-02915-8 DOI 10.1007/978-3-642-02915-8 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2009935962 © Springer-Verlag Berlin Heidelberg 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: WMXDesign GmbH, Heidelberg Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Electroanalytical Methods Fritz Scholz dedicates this book to the memory of his late parents Anneliese and Herbert Scholz Preface to the Second Edition The authors are pleased to present here the second edition of the book “Electroanalytical Methods.