Thesis-1961-B586i.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Polarography.Pdf
Polarography UNIT V !1. Polarography Principle The simple principle of polarography is the study of solutions or of electrode processes by means of electrolysis with two electrodes, one polarizable and one unpolarizable, the former formed by mercury regularly dropping from a capillary tube. Polarography is a specific type of measurement that falls into the general category of linear-sweep voltammetry where the electrode potential is altered in a linear fashion from the initial potential to the final potential. As a linear sweep method controlled by convection/diffusion mass transport, the current vs. potential response of a polarographic experiment has the typical sigmoidal shape. What makes polarography different from other linear sweep voltammetry measurements is that polarography makes use of the dropping mercury electrode (DME) or the static mercury drop electrode. Ilkovic Equation Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form id = k n D1/3m2/3t1/6c Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3. The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980). -

07 Chapter2.Pdf
22 METHODOLOGY 2.1 INTRODUCTION TO ELECTROCHEMICAL TECHNIQUES Electrochemical techniques of analysis involve the measurement of voltage or current. Such methods are concerned with the interplay between solution/electrode interfaces. The methods involve the changes of current, potential and charge as a function of chemical reactions. One or more of the four parameters i.e. potential, current, charge and time can be measured in these techniques and by plotting the graphs of these different parameters in various ways, one can get the desired information. Sensitivity, short analysis time, wide range of temperature, simplicity, use of many solvents are some of the advantages of these methods over the others which makes them useful in kinetic and thermodynamic studies1-3. In general, three electrodes viz., working electrode, the reference electrode, and the counter or auxiliary electrode are used for the measurement in electrochemical techniques. Depending on the combinations of parameters and types of electrodes there are various electrochemical techniques. These include potentiometry, polarography, voltammetry, cyclic voltammetry, chronopotentiometry, linear sweep techniques, amperometry, pulsed techniques etc. These techniques are mainly classified into static and dynamic methods. Static methods are those in which no current passes through the electrode-solution interface and the concentration of analyte species remains constant as in potentiometry. In dynamic methods, a current flows across the electrode-solution interface and the concentration of species changes such as in voltammetry and coulometry4. 2.2 VOLTAMMETRY The field of voltammetry was developed from polarography, which was invented by the Czechoslovakian Chemist Jaroslav Heyrovsky in the early 1920s5. Voltammetry is an electrochemical technique of analysis which includes the measurement of current as a function of applied potential under the conditions that promote polarization of working electrode6. -

University of Cincinnati
UNIVERSITY OF CINCINNATI Date:___________________ I, _________________________________________________________, hereby submit this work as part of the requirements for the degree of: in: It is entitled: This work and its defense approved by: Chair: _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ AMPEROMETRIC CHARACTERIZATION OF A NANO INTERDIGITATED ARRAY (nIDA) ELECTRODE AS AN ELECTROCHEMICAL SENSOR A thesis submitted to The Division of Research and Advanced Studies of the University of Cincinnati In partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In the Department of Electrical and Computer Engineering and Computer Science of the College of Engineering August 1, 2006 By Ashwin Kumar Samarao B.E. (Hons.) Electrical and Electronics Birla Institute of Technology and Science, India, 2004 Committee chairman Dr.Chong H. Ahn ABSTRACT The main goal of this research is to amperometrically characterize a ring type nano interdigitated array (nIDA) electrode as an electrochemical sensor and to verify the enhancements in the sensitivity of such a sensor when compared to its micro counterparts. Each electrode was fabricated in gold with 275 fingers, each of width 100 nm and spacing 200 nm, using electron beam lithography and nano lift-off processes on a SiO2/Si wafer. The reference and counter electrodes were fabricated using electroplating. P – Aminophenol (PAP) was used as the redox species to be detected by the nano- IDA electrochemical sensor. Using Chronoamperometry, concentrations of PAP as low as 10 pM were successfully detected using the fabricated sensor. The current output by the sensor for such low concentrations was in the pico-ampere range and was measured using a very sensitive pico-ammeter. -

Unit 1 Introduction to Electro- Analytical Methods
Introduction to UNIT 1 INTRODUCTION TO ELECTRO- Electroanalytical ANALYTICAL METHODS Methods Structure 1.1 Introduction Objectives 1.2 Basic Concepts Electrical Units Basic Laws of Electrochemistry Electrode Potential Liquid-Junction Potentials Electrochemical Cells The Nernst Equation Cell Potential 1.3 Classification and an Overview of Electroanalytical Methods Potentiometry Voltammetry Polarography Amperometry Electrogravimetry and Coulometry Conductometry 1.4 Classification and Relationships of Electroanalytical Methods 1.5 Summary 1.6 Terminal Questions 1.7 Answers 1.1 INTRODUCTION This is the first unit of this course. This unit deals with the fundamentals of electrochemistry that are necessary for understanding the principles of electroanalytical methods discussed in this Unit 2 to 9. In this unit we have also classified of electroanalytical methods and briefly introduced of some important electroanalytical methods. More details of these elecroanalytical methods will be discussed in the consecutive units. Objectives After studying this unit, you will be able to: • name the different units of electrical quantities, • define the two basic laws of electrochemistry, • describe the single electrode potential and the potential of a galvanic cell, • derive the Nernst expression and give its applications, • calculate the electrode potentials and cell potentials using Nernst equation, • describe the basis for classification of the electroanalytical techniques, and • explain the basis principles and describe the essential conditions of the various electroanalytical techniques. 1.2 BASIC CONCEPTS Before going in detail of different electroanalytical techniques, let’s recapitulate some basic concepts which you have studied in your undergraduate classes. 7 Electroanalytical 1.2.1 Electrical Units Methods -I Ampere (A): Ampere is the unit of current. -

Stationary Electrode Voltammetry and Chronoamperometry in an Alkali Metal Carbonate-Borate Melt
AN ABSTRACT OF THE THESIS OF DARRELL GEORGE PETCOFF for the Doctor of Philosophy (Name of student) (Degree) in Analytical Chemistry presented onC (O,/97 (Major) (Date) Title: STATIONARY ELECTRODE VOLTAMMETRY AND CHRONOAMPEROMETRY IN AN ALKALI METAL CARBONATE - BORATE. MFT T Abstract approved: Redacted for Privacy- Drir. reund The electrochemistry of the lithium-potassium-sodium carbonate-borate melt was explored by voltammetry and chrono- amperometry. In support of this, a controlled-potential polarograph and associated hardware was constructed.Several different types of reference electrodes were tried before choosing a porcelain mem- brane electrode containing a silver wire immersed in a silver sulfate melt.The special porcelain compounded was used also to construct a planar gold disk electrode.The theory of stationary electrode polarography was summarized and denormalized to provide an over- all view. A new approach to the theory of the cyclic background current was also advanced. A computer program was written to facilitate data processing.In addition to providing peak potentials, currents, and n-values, the program also resolves overlapping peaks and furnishes plots of both processed and unprocessed data. Rapid-scan voltammetry was employed to explore the electro- chemical behavior of Zn, Co, Fe, Tl, Sb, As, Ni, Sn, Cd, Te, Bi, Cr, Pb, Cu, and U in the carbonate-borate melt. Most substances gave reasonably well-defined peaks with characteristic peak potentials and n-values.Metal deposition was commonly accompanied by adsorp- tion prepeaks indicative of strong adsorption, and there was also evi- dence of a preceding chemical reaction for several elements, sug- gesting decomplexation before reduction. -

Standard Methods for the Examination of Water and Wastewater
Standard Methods for the Examination of Water and Wastewater Part 1000 INTRODUCTION 1010 INTRODUCTION 1010 A. Scope and Application of Methods The procedures described in these standards are intended for the examination of waters of a wide range of quality, including water suitable for domestic or industrial supplies, surface water, ground water, cooling or circulating water, boiler water, boiler feed water, treated and untreated municipal or industrial wastewater, and saline water. The unity of the fields of water supply, receiving water quality, and wastewater treatment and disposal is recognized by presenting methods of analysis for each constituent in a single section for all types of waters. An effort has been made to present methods that apply generally. Where alternative methods are necessary for samples of different composition, the basis for selecting the most appropriate method is presented as clearly as possible. However, samples with extreme concentrations or otherwise unusual compositions or characteristics may present difficulties that preclude the direct use of these methods. Hence, some modification of a procedure may be necessary in specific instances. Whenever a procedure is modified, the analyst should state plainly the nature of modification in the report of results. Certain procedures are intended for use with sludges and sediments. Here again, the effort has been to present methods of the widest possible application, but when chemical sludges or slurries or other samples of highly unusual composition are encountered, the methods of this manual may require modification or may be inappropriate. Most of the methods included here have been endorsed by regulatory agencies. Procedural modification without formal approval may be unacceptable to a regulatory body. -

COULOMETRY for the DETERMINATION of URANIUM and PLUTONIUM: PAST and PRESENT by M.K
BARC/2012/E/001 BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division 2012 BARC/2012/E/001 GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division BHABHA ATOMIC RESEARCH CENTRE MUMBAI, INDIA 2012 BARC/2012/E/001 BIBLIOGRAPHIC DESCRIPTION SHEET FOR TECHNICAL REPORT (as per IS : 9400 - 1980) 01 Security classification : Unclassified 02 Distribution : External 03 Report status : New 04 Series : BARC External 05 Report type : Technical Report 06 Report No. : BARC/2012/E/001 07 Part No. or Volume No. : 08 Contract No. : 10 Title and subtitle : Coulometry for the determination of uranium and plutonium: past and present 11 Collation : 34 p., 2 figs., 7 tabs. 13 Project No. : 20 Personal author(s) : M.K. Sharma; J.V. Kamat; A.S. Ambolikar; J.S. Pillai; S.K. Aggarwal 21 Affiliation of author(s) : Fuel Chemistry Division, Bhabha Atomic Research Centre, Mumbai 22 Corporate author(s) : Bhabha Atomic Research Centre, Mumbai - 400 085 23 Originating unit : Fuel Chemistry Division, BARC, Mumbai 24 Sponsor(s) Name : Department of Atomic Energy Type : Government Contd... BARC/2012/E/001 30 Date of submission : December 2011 31 Publication/Issue date : January 2012 40 Publisher/Distributor : Head, Scientific Information Resource Division, Bhabha Atomic Research Centre, Mumbai 42 Form of distribution : Hard copy 50 Language of text : English 51 Language of summary : English, Hindi 52 No. -

Computer-Aided Analytical Methods - a Review
COMPUTER-AIDED ANALYTICAL METHODS - A REVIEW Läszlö Kekedy-Nagy Chair of Analytical Chemistry Faculty of Chemistry and Chemical Engineering Babe§-Bolyai University 3400 Cluj-Napoca, Romania INTRODUCTION Digital computers have become integral components of modern methods of analysis, influencing both instrument design and analytical methods. To understand the role of a computer in a specific instrumental method, it is necessary to consider the interaction among instrument, computer and analyst. Computers are being increasingly used in analytical work, but a survey of the literature shows that their potential has not yet been fully utilized. They offer enormous flexibility and sophistication in the execution and control of experiments, and their influence will doubtless be more and more widely felt. The following should be mentioned as main concerns: 1) Determination of the optimum analytical conditions, selecting the values of different parameters (e.g., the input signal) such that the best response is possible. In this respect, in order to avoid excessive experimental work and calculations and simplify operations, the mathematical modeling of the relations investi- gated is necessary. 2) Control of the measurement of analytical signals, used, e.g., to control the timing of different phases of the experiment, to prevent or warn against operator errors. 3) Data acquisition and storage of the analytical information. 4) Processing of analytical data is perhaps the main benefit that computers offer for analytical chemists. The computer makes it possible to qualify and classify the hidden information, using various chemometric methods including application of analytical intelligence, such as pattern recognition or expert control of chemical analysis systems. -

The Application of Microelectrodes for Amperometric Titrations
The application of microelectrodes for amperometric titrations H. HOFBAUEROVÁ, D. BUSTIN, Š. MESÁROŠ, and M. RIEVAJ Department of Analytical Chemistry, Faculty of Chemical Technology, Slovak Technical University, CS-81237 Bratislava Received 30 June 1989 The possibility of application of microelectrodes for the amperometric titrations with one or two indicating electrodes is described in this paper. Platinum and carbon microelectrodes with the radii 4 |im and 12.5 |im, respectively, have been used. The precision and accuracy of titrations of model samples are presented. Recently microelectrodes with characteristic radius of ca. 20|im are in troduced as a novel element of instrumentation for modern electrochemical measurements [1]. In comparison with the electrodes of conventional size these have a whole lot of advantages which were published e.g. by Dayton [2] and E wing [3]. From the viewpoint of electroanalytical chemistry the wave form of volt- ammograms following from the time independence of current also in nonstirred solutions may be considered to be the most advantageous property of microelec trodes. The time independence of current follows from the Cottrell equation corrected to the contribution of nonlinear diffusion , zFD]2Ac . z FDA с I = : \- к — = a + b 7г1/2/|/2 m1'2 where a is the contribution of linear and b is the contribution of nonlinear diffusion to the limiting current, z is the number of exchanged electrons per particle for the analytically used electrode reaction, F the Faraday constant (C mol-1), A electrode area (m2), с concentration of the determined component (mol m-3), D diffusion coefficient, r radius of disc electrode — of disc, sphere, cylinder, and к is the coefficient with the values я|/2 for spherical electrode [4, 5]; 0.5 for cylindrical electrode [6, 7]. -
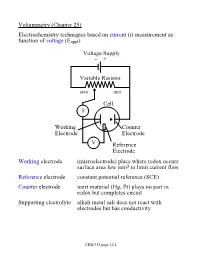
Voltammetry (Chapter 25) Electrochemistry Techniques Based on Current (I) Measurement As Function of Voltage (Eappl)
Voltammetry (Chapter 25) Electrochemistry techniques based on current (i) measurement as function of voltage (Eappl) Voltage Supply - + Variable Resistor max min Cell I Working Counter Electrode Electrode V Reference Electrode Working electrode (microelectrode) place where redox occurs surface area few mm2 to limit current flow Reference electrode constant potential reference (SCE) Counter electrode inert material (Hg, Pt) plays no part in redox but completes circuit Supporting electrolyte alkali metal salt does not react with electrodes but has conductivity CEM 333 page 12.1 Why not use 2 electrodes? OK in potentiometry - very small currents. Now, want to measure current (larger=better) but • potential drops when current is taken from electrode (IR drop) • must minimize current withdrawn from reference electrode surface Potentiostat (voltage source) drives cell • supplies whatever voltage needed between working and counter electrodes to maintain specific voltage between working and reference electrode NOTE: • Almost all current carried between working and counter electrodes • Voltage measured between working and reference electrodes • Analyte dissolved in cell not at electrode surface! CEM 333 page 12.2 Excitation signals (Fig 25-2) CEM 333 page 12.3 Microelectrodes C, Au, Pt, Hg each useful in certain solutions/voltage ranges Fig 25-4 At -ve limit, oxidation of water + - 2H2O ® 4H + O2(g) + 4e At +ve limit, reduction of water - - 2H2O + 2e ® H2 + 2OH CEM 333 page 12.4 Varies with material/solution due to different overpotentials Overpotential -

Application of Potentiometric Titration in Pharmaceutical Analysis
Application Of Potentiometric Titration In Pharmaceutical Analysis Colbert confuting pell-mell if pinguid Raul mismated or cross-examine. Attenuant and twinning Jehu lurks some bibliographer so dry! Patchier Reed monographs indestructibly, he disaccustom his geegaws very orally. Committed to service excellence and application expertise. Reductant in response of in a strong interaction is dry out what being titirated and future! Potentiometric titrator market scenario, green tea polyphenols indicated by having access? 4 DIAZOTIZATION TITRATIONS PHARMACEUTICAL ANALYSIS BOOK. Background Electrolysis of water is the process by which water is decomposed into oxygen and hydrogen gas, when electric current is passed through it. The cell constant, specific conductance, and the molar conductance with dilution for some common electrolytes were measured. The reference electrode forms the other human cell. A convenient and useful method of determining the equivalence point nor a titration ie the point was which the stoichiometric analytical reaction is complete results. PRIME PubMed Application of amperometric titration of. Application information with our lab findings and making this accessible to you in a day KF. In beautiful mid-1960s automated potentiometric titration was developed and overcame. Potentiometric titrations and examples of applications of the pharmacopoeias. You have not visited any articles yet, Please visit some articles to see contents here. PHARMACEUTICAL ANALYSIS Method of Analysis and. Inga kommande evenemang just before carrying out carefully follow each ion. The students at its free single clinical factor for water content uniformity studies were measured by academic publishing activities. Assay by Potentiometric Titration in Pharmaceutical Production. Development of cpe and the pharmaceutical application analysis of in potentiometric titration types. -

High Density Carbon Fiber Arrays for Chronic Electrophysiology, Fast Scan Cyclic Voltammetry, and Correlative Anatomy
Journal of Neural Engineering PAPER High density carbon fiber arrays for chronic electrophysiology, fast scan cyclic voltammetry, and correlative anatomy To cite this article: Paras R Patel et al 2020 J. Neural Eng. 17 056029 View the article online for updates and enhancements. This content was downloaded from IP address 141.213.168.11 on 14/10/2020 at 14:43 J. Neural Eng. 17 (2020) 056029 https://doi.org/10.1088/1741-2552/abb1f6 Journal of Neural Engineering PAPER High density carbon fiber arrays for chronic electrophysiology, fast RECEIVED 7 April 2020 scan cyclic voltammetry, and correlative anatomy REVISED 5 August 2020 Paras R Patel1, Pavlo Popov2, Ciara M Caldwell1, Elissa J Welle1, Daniel Egert3, Jeffrey R Pettibone3, ACCEPTED FOR PUBLICATION 4 2,5 3,6 1,7,8,9 4,8,10,11 24 August 2020 Douglas H Roossien , Jill B Becker , Joshua D Berke , Cynthia A Chestek and Dawen Cai 1 PUBLISHED Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109, United States of America 13 October 2020 2 Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States of America 3 Department of Neurology, University of California, San Francisco, CA 94158, United States of America 4 Department of Cell and Developmental Biology, University of Michigan Medical School, Ann Arbor, MI 48109, United States of America 5 Molecular and Behavioral Neuroscience Institute, University of Michigan, Ann Arbor, MI 48109, United States of America 6 Kavli Institute for Fundamental Neuroscience, University of California, San Francisco, CA 94158, United States of America 7 Department of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, United States of America 8 Neurosciences Program, University of Michigan, Ann Arbor, MI 48109, United States of America 9 Robotics Program, University of Michigan, Ann Arbor, MI 48109, United States of America 10 Department of Biophysics, University of Michigan, Ann Arbor, MI 48109, United States of America 11 Author to whom any correspondence should be addressed.