Estimation of Tegaserod Maleate by Differential Pulse Polarography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Polarography.Pdf
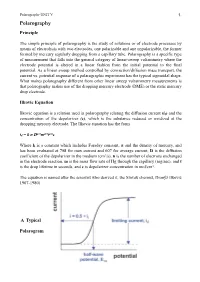
Polarography UNIT V !1. Polarography Principle The simple principle of polarography is the study of solutions or of electrode processes by means of electrolysis with two electrodes, one polarizable and one unpolarizable, the former formed by mercury regularly dropping from a capillary tube. Polarography is a specific type of measurement that falls into the general category of linear-sweep voltammetry where the electrode potential is altered in a linear fashion from the initial potential to the final potential. As a linear sweep method controlled by convection/diffusion mass transport, the current vs. potential response of a polarographic experiment has the typical sigmoidal shape. What makes polarography different from other linear sweep voltammetry measurements is that polarography makes use of the dropping mercury electrode (DME) or the static mercury drop electrode. Ilkovic Equation Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form id = k n D1/3m2/3t1/6c Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3. The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980). -

07 Chapter2.Pdf
22 METHODOLOGY 2.1 INTRODUCTION TO ELECTROCHEMICAL TECHNIQUES Electrochemical techniques of analysis involve the measurement of voltage or current. Such methods are concerned with the interplay between solution/electrode interfaces. The methods involve the changes of current, potential and charge as a function of chemical reactions. One or more of the four parameters i.e. potential, current, charge and time can be measured in these techniques and by plotting the graphs of these different parameters in various ways, one can get the desired information. Sensitivity, short analysis time, wide range of temperature, simplicity, use of many solvents are some of the advantages of these methods over the others which makes them useful in kinetic and thermodynamic studies1-3. In general, three electrodes viz., working electrode, the reference electrode, and the counter or auxiliary electrode are used for the measurement in electrochemical techniques. Depending on the combinations of parameters and types of electrodes there are various electrochemical techniques. These include potentiometry, polarography, voltammetry, cyclic voltammetry, chronopotentiometry, linear sweep techniques, amperometry, pulsed techniques etc. These techniques are mainly classified into static and dynamic methods. Static methods are those in which no current passes through the electrode-solution interface and the concentration of analyte species remains constant as in potentiometry. In dynamic methods, a current flows across the electrode-solution interface and the concentration of species changes such as in voltammetry and coulometry4. 2.2 VOLTAMMETRY The field of voltammetry was developed from polarography, which was invented by the Czechoslovakian Chemist Jaroslav Heyrovsky in the early 1920s5. Voltammetry is an electrochemical technique of analysis which includes the measurement of current as a function of applied potential under the conditions that promote polarization of working electrode6. -

A Study of the Electrodynamometer
A°)tud^Of The EkclrodvnamomeUr. A STUDY OF THE ELECTROD YN AMOM ETER BY ELEANOR FRANCES SEILER B. A. University of Denver, 1913 M.A. University of Denver, 1914 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF ARTS IN PHYSICS IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1916 Digitized by the Internet Archive in 2013 http://archive.org/details/studyofelectrodyOOseil UNIVERSITY OF ILLINOIS co THE GRADUATE SCHOOL Oo CM . Jnnfl J.. .191 6 1 HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPER- VISION BY BLHABQR JKANQBS fiF!TT,Blft ENTITLED A....S.T.UDY.....QE......THE....ELE.CT.RanY.NAIiOIAE.TER.. BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF MASjES.R.....QE....S.C.IEif.C.S In Charge of Thesis Head of Department Recommendation concurred in :* Committee on Final Examination* "Required for doctor's degree but not for master's. 343113 UIUC V J 5 TABLE OF CONTENTS I INTRODUCTION II DESCRIPTION 0? APPARATUS I - General I - Specific 5 - The Spring g - Best Shape for Movable Coil 7 - Other Types of Electrodynamoraeters 12 - Rowland Elec trodynamome ter 12 - Absolute Electrodynarnometer 14 - Lord Kelvin Current Balance 1 III THEORY OF THE ELECTRODYNAMOMETER 1 - General J5 - When Used as an Ammeter 16 - When Usee} as a Wattmeter with Direct Current 19 - When U3ed as a Wattmeter with Alternating Current _ gj - When Used as a Voltmeter 23 - Use 13 a Method for Measuring Hysteresis . 24 IV PRELIMINARY WORK 04 - Calibrations 24 - Mechanical Difficulties 37 V MANIPULATION 38 VI DISCUSSION OF RESULTS 48 - Sources of Error 49 : ! I INTRODUCTION In thl3 investigation the primary object was to determine the wording conditions under which the beet results may be expected when the classic Siemens type of elec trodynaraometer is used as a watt- meter. -

The Silver Voltameter—Part Iv
.. 1 THE SILVER VOLTAMETER—PART IV THIRD SERIES OF QUANTITATIVE EXPERIMENTS AND SPECIAL INVESTIGATIONS By E. B. Rosa, G. W. Vinal, and A. S. McDanlel CONTENTS Page I. Introduction 476 II. DETERMINATION OP VOLTAGE OF THE WESTON NoRMAIv Cei.I< 477 A. Basis of reference for current and voltage measurements 478 1. Weston normal cells 478 2. Resistances 478 B. Meaning of the term " International volts" 478 C. Voltameters and materials 479 D. Tables of results 481 1. Corrections for acid present 482 2. Resultsof the porous pot and Smith's new-form voltameters. 485 3. Results of other forms of voltameters 492 4. Miscellaneous results 493 III. Speciai, Investigations 497 A. On the agreement of various forms 497 1 Comparative results 497 2 Possible explanations of observed differences 498 B. On the accuracy of the measurements. 501 C. Reliability of iodeosine as an indicator 502 D. Effect of acid 503 1 Effect on impure solutions 503 2 Nature of the action of acid 505 E. Influence of septa other than filter paper 508 1. Silk 508 2 Poroiis pots 510 3. Electrostenolysis 514 F. Purity of the silver deposit 516 1. Constancy of any error due to inclusions 516 2. Previous determinations of inclusions 517 3. Experiments on inclusions 519 (a) Detection of silver nitrate 519 (b) Estimation of included water and volatile material 52 (c) Estimation of organic material 522 (d) Titration of silver deposits 523 (e) Conclusion 523 475 476 Bulletin of the Bureau of Standards {Voi.io III. SpeciaIv Investigations—Continued. Page, G. Reversibility of the silver voltameter 523 H. -

Unit 1 Introduction to Electro- Analytical Methods
Introduction to UNIT 1 INTRODUCTION TO ELECTRO- Electroanalytical ANALYTICAL METHODS Methods Structure 1.1 Introduction Objectives 1.2 Basic Concepts Electrical Units Basic Laws of Electrochemistry Electrode Potential Liquid-Junction Potentials Electrochemical Cells The Nernst Equation Cell Potential 1.3 Classification and an Overview of Electroanalytical Methods Potentiometry Voltammetry Polarography Amperometry Electrogravimetry and Coulometry Conductometry 1.4 Classification and Relationships of Electroanalytical Methods 1.5 Summary 1.6 Terminal Questions 1.7 Answers 1.1 INTRODUCTION This is the first unit of this course. This unit deals with the fundamentals of electrochemistry that are necessary for understanding the principles of electroanalytical methods discussed in this Unit 2 to 9. In this unit we have also classified of electroanalytical methods and briefly introduced of some important electroanalytical methods. More details of these elecroanalytical methods will be discussed in the consecutive units. Objectives After studying this unit, you will be able to: • name the different units of electrical quantities, • define the two basic laws of electrochemistry, • describe the single electrode potential and the potential of a galvanic cell, • derive the Nernst expression and give its applications, • calculate the electrode potentials and cell potentials using Nernst equation, • describe the basis for classification of the electroanalytical techniques, and • explain the basis principles and describe the essential conditions of the various electroanalytical techniques. 1.2 BASIC CONCEPTS Before going in detail of different electroanalytical techniques, let’s recapitulate some basic concepts which you have studied in your undergraduate classes. 7 Electroanalytical 1.2.1 Electrical Units Methods -I Ampere (A): Ampere is the unit of current. -

Recommendation for Use Hofmann Voltameter for Educational Purposes
Glaswarenfabrik Tel. +49 (0) 9779 808 0 Karl Hecht Fax +49 (0) 9779 808 88 GmbH & Co KG Email: [email protected] Stettener Straße 22-24 Internet: www.assistent.eu 97647 Sondheim/Rhön VAT No. DE132198535 Germany Recommendation for use Hofmann voltameter for educational purposes Product number: 44285010 and 44286010 A water decomposition apparatus is used for the demonstration of electrolysis, i. e. for the decomposition of water into hydrogen and oxygen by means of electric current. The apparatus is consisting of a glass tube with pear-shaped funnel and two lateral measuring tubes 60 ml : 0.2 ml with glass stopcocks. There are two versions available: Product number 44285010 Spare parts Glass part No. 44287010 Metal tripod 2 Clamps No. 40870010 2 Platinum electrodes No. 44288010 Product number 44286010 Spare parts Glass part No. 44287010 Metal tripod 2 Clamps No. 40870010 2 Carbon electrodes No. 44289010 Experimental setup and test procedure Attach the voltameter with two clamps to the support. Insert the carbon or platinum electrodes into the measuring tubes. The stoppers have to be firmly in position in order to avoid leakage of liquid. For safety reasons place the apparatus into a glass tray. Pour in the electrolyte through the funnel while stopcocks are both open to let the air escape. Close the stopcocks as soon as the electrolyte has reached the level of stopcock bores. Connect the electrodes and the pole terminals at the tripod by means of two short connecting cables. Connect a DC voltage source and supply with electrical energy. The required direct voltage is about 10 V to 20 V at max. -

Stationary Electrode Voltammetry and Chronoamperometry in an Alkali Metal Carbonate-Borate Melt
AN ABSTRACT OF THE THESIS OF DARRELL GEORGE PETCOFF for the Doctor of Philosophy (Name of student) (Degree) in Analytical Chemistry presented onC (O,/97 (Major) (Date) Title: STATIONARY ELECTRODE VOLTAMMETRY AND CHRONOAMPEROMETRY IN AN ALKALI METAL CARBONATE - BORATE. MFT T Abstract approved: Redacted for Privacy- Drir. reund The electrochemistry of the lithium-potassium-sodium carbonate-borate melt was explored by voltammetry and chrono- amperometry. In support of this, a controlled-potential polarograph and associated hardware was constructed.Several different types of reference electrodes were tried before choosing a porcelain mem- brane electrode containing a silver wire immersed in a silver sulfate melt.The special porcelain compounded was used also to construct a planar gold disk electrode.The theory of stationary electrode polarography was summarized and denormalized to provide an over- all view. A new approach to the theory of the cyclic background current was also advanced. A computer program was written to facilitate data processing.In addition to providing peak potentials, currents, and n-values, the program also resolves overlapping peaks and furnishes plots of both processed and unprocessed data. Rapid-scan voltammetry was employed to explore the electro- chemical behavior of Zn, Co, Fe, Tl, Sb, As, Ni, Sn, Cd, Te, Bi, Cr, Pb, Cu, and U in the carbonate-borate melt. Most substances gave reasonably well-defined peaks with characteristic peak potentials and n-values.Metal deposition was commonly accompanied by adsorp- tion prepeaks indicative of strong adsorption, and there was also evi- dence of a preceding chemical reaction for several elements, sug- gesting decomplexation before reduction. -

Thesis-1961-B586i.Pdf
INVESTIGATION OF SOME POSSIBILITIES FOR AMPEROMETRIC TITRATION OF CERTAIN METAL IONS WITH OXINE By Donald George Biechler I I Bachelor of Science University of Wisconsin Madison, Wisconsin 1956 Submitted to the faculty of the Graduate School of the Oklahoma State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May, 1961 INVESTIGATION OF SOME POSSIBILITIES FOR AMPEROMEI'RIC TITRATION OF CERTAIN MEI'AL IONS WITH OXINE Thesis Approved: Thesis Adviser i i OKLAHOMA STATE UNIVERSITY llBRARY JAN 2 1962 PREFACE Oxine (8-hydroxyquinoline) is most generally used in analytical chemistry as a precipitant for metals and is known to form water- insoluble chelates with better than thirty metal ions (3). There exists in solutions of oxine a tautomeric equilibrium of the fol- lowing type: C C C C /~/'\ ,c/""/~ C C f ij 1 I II I C C C C C C ~/"'/C N ~/C "+/N r . _I I 0 H 0--------H Chelation of a metal ion involves replacement of the proton and for- mation of a coordinate bond with the nitrogen to form a stable 5 membered ring compound. Thus nickel, a bivalent cation, would form a compound with the following structure: 4810 90 iii iv The oxinates can be ignited and weighed as such or they may be further ignited to the metal oxides and then weighed. Alternately the oxinates may be dissolved in acid and quantitatively brominated (7)0 Considering the number of metal ions that are precipitated by oxine, it seemed that possibly more use could be made of the reagent in volumetric analysis. -

The High-Precision Thermometric and Coulometric Assay of Primary Standard 4-Aminopyridine William Charles Hoyle Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1973 The high-precision thermometric and coulometric assay of primary standard 4-aminopyridine William Charles Hoyle Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons Recommended Citation Hoyle, William Charles, "The high-precision thermometric and coulometric assay of primary standard 4-aminopyridine " (1973). Retrospective Theses and Dissertations. 5017. https://lib.dr.iastate.edu/rtd/5017 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

INDIAN INSTITUTE of TECHNOLOGY KANPUR Department of Civil Engineering Environmental Engineering and Management Programme Kanpur-208016, INDIA
INDIAN INSTITUTE OF TECHNOLOGY KANPUR Department of Civil Engineering Environmental Engineering and Management Programme Kanpur-208016, INDIA Dr. Vinod Tare Professor Tel : +91 512 259 7792 (O), Fax : +91 512 259 7395 (O); E-mail : [email protected] Enquiry Letter No. CE/EEM/2013/NC/VA/10 Date: Aug 22, 2013 Last Date: September 9, 2013 Letter Requesting Quotation for Voltameter Quotation is sought for a Voltameter with minimum specifications given in the list below. The quotation has to be submitted in two separately sealed envelopes, one containing the technical specifications and marked as “Technical Quote” on the envelope and the other containing the price and marked as the “Price Quote” on the envelope. Please apply the discounts admissible to the educational institutes such as IIT Kanpur and also note that IIT Kanpur is exempted from paying duties for imported items. Minimum Specification Required: • Voltameter: determination of transition metals like copper, tin ,vanadium , mercury and other metals based on Voltammetry principle with wide-band low-noise amplifier, potentiostat and galvanostat. Voltage Range: ± 5 V, Output Range: ± 12 V, Current Range: ± 80 mA with measurement resolution of 10nA to 10mA, sweep rate of 1mV/s to 3V/s and/or 35V/s • Electrode System: multimode SMDE, HMDE and DME, choice of multiple drop sizes, should minimize mercury requirement. Rotating disc electrode facility and various solid-state electrodes like Gold, Silver, Glassy Carbon, Platinum and Graphite. • Instrument Control and Analysis: Computer and Software must be provided for fully computer controlled operation, data acquisition and quantitative analysis. Software must be able to analyse by following voltametric techniques: Direct Current (DC), Sampled DC, Differential Pulse (DP), Square-wave (SQW), Alternating Current (AC) 1st and 2nd harmonic, phase selective, Cyclic Voltammetry (CV), Potentometric Stripping Analysis (PSA), Normal Pulse (NP) and Constant Current Potentiometric Stripping Analysis (CCPSA). -
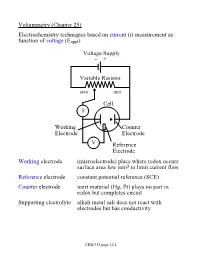
Voltammetry (Chapter 25) Electrochemistry Techniques Based on Current (I) Measurement As Function of Voltage (Eappl)
Voltammetry (Chapter 25) Electrochemistry techniques based on current (i) measurement as function of voltage (Eappl) Voltage Supply - + Variable Resistor max min Cell I Working Counter Electrode Electrode V Reference Electrode Working electrode (microelectrode) place where redox occurs surface area few mm2 to limit current flow Reference electrode constant potential reference (SCE) Counter electrode inert material (Hg, Pt) plays no part in redox but completes circuit Supporting electrolyte alkali metal salt does not react with electrodes but has conductivity CEM 333 page 12.1 Why not use 2 electrodes? OK in potentiometry - very small currents. Now, want to measure current (larger=better) but • potential drops when current is taken from electrode (IR drop) • must minimize current withdrawn from reference electrode surface Potentiostat (voltage source) drives cell • supplies whatever voltage needed between working and counter electrodes to maintain specific voltage between working and reference electrode NOTE: • Almost all current carried between working and counter electrodes • Voltage measured between working and reference electrodes • Analyte dissolved in cell not at electrode surface! CEM 333 page 12.2 Excitation signals (Fig 25-2) CEM 333 page 12.3 Microelectrodes C, Au, Pt, Hg each useful in certain solutions/voltage ranges Fig 25-4 At -ve limit, oxidation of water + - 2H2O ® 4H + O2(g) + 4e At +ve limit, reduction of water - - 2H2O + 2e ® H2 + 2OH CEM 333 page 12.4 Varies with material/solution due to different overpotentials Overpotential -

The Inclusion of Electrolyte by the Deposit in the Silver Voltameter
ELECTROLYTE INCLUSION IN SILVER VOLTAMETER. 7 at the changes for the better that have taken place within my life time. Chemical research is in a healthy condition in our country and the signs of future growth are most promising. May I add in conclusion that, though circumstances have kept me out of the field of chemical work for some years past, I now see my way clear to entering that field again, and I can think of nothing that could give me greater pleasure than the prospect of taking up the work in chemistry that I had to abandon thirteen years ago. It is a little late for me to begin again, but I believe that I shall yet be able to experience some of the joys that came to me so abundantly in the past while struggling with my old, inanimate labora- tory friends. The transformation from university president to chemist is complete, and I rejoice. JOHNS HOPKINS UNIVERSITY, BALTIMORE, MD. [CONTRIBUTIONFROM THE WOLCOTTGIBBS MEMORIALLABORATORY OF HARVARD UNIVERSITY.] THE INCLUSION OF ELECTROLYTE BY THE DEPOSIT IN THE SILVER VOLTAMETER. BY T. W. RICHARDSAND F. 0. ANDEREGO. Received October 31, 1914. I. Introduction. The silver voltameter (or better, coulometer) is an instrument of such great importance in fhe exact measurement of the electrical quantity that its study by many investigators is highly desirable; therefore, the wide- spread attention which it has received during recent years is gratifying. Since the classical researches of Lord Rayleigh and Mrs. Sidgwick' and F. and W. Kohlrausch,2 carried out between 1880 and 1883, the sub- ject has been studied in may places and from many points of view.