Simultaneous Coulcmetric Analtsis Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of the Electrodynamometer
A°)tud^Of The EkclrodvnamomeUr. A STUDY OF THE ELECTROD YN AMOM ETER BY ELEANOR FRANCES SEILER B. A. University of Denver, 1913 M.A. University of Denver, 1914 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF ARTS IN PHYSICS IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1916 Digitized by the Internet Archive in 2013 http://archive.org/details/studyofelectrodyOOseil UNIVERSITY OF ILLINOIS co THE GRADUATE SCHOOL Oo CM . Jnnfl J.. .191 6 1 HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPER- VISION BY BLHABQR JKANQBS fiF!TT,Blft ENTITLED A....S.T.UDY.....QE......THE....ELE.CT.RanY.NAIiOIAE.TER.. BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF MASjES.R.....QE....S.C.IEif.C.S In Charge of Thesis Head of Department Recommendation concurred in :* Committee on Final Examination* "Required for doctor's degree but not for master's. 343113 UIUC V J 5 TABLE OF CONTENTS I INTRODUCTION II DESCRIPTION 0? APPARATUS I - General I - Specific 5 - The Spring g - Best Shape for Movable Coil 7 - Other Types of Electrodynamoraeters 12 - Rowland Elec trodynamome ter 12 - Absolute Electrodynarnometer 14 - Lord Kelvin Current Balance 1 III THEORY OF THE ELECTRODYNAMOMETER 1 - General J5 - When Used as an Ammeter 16 - When Usee} as a Wattmeter with Direct Current 19 - When U3ed as a Wattmeter with Alternating Current _ gj - When Used as a Voltmeter 23 - Use 13 a Method for Measuring Hysteresis . 24 IV PRELIMINARY WORK 04 - Calibrations 24 - Mechanical Difficulties 37 V MANIPULATION 38 VI DISCUSSION OF RESULTS 48 - Sources of Error 49 : ! I INTRODUCTION In thl3 investigation the primary object was to determine the wording conditions under which the beet results may be expected when the classic Siemens type of elec trodynaraometer is used as a watt- meter. -

The Silver Voltameter—Part Iv
.. 1 THE SILVER VOLTAMETER—PART IV THIRD SERIES OF QUANTITATIVE EXPERIMENTS AND SPECIAL INVESTIGATIONS By E. B. Rosa, G. W. Vinal, and A. S. McDanlel CONTENTS Page I. Introduction 476 II. DETERMINATION OP VOLTAGE OF THE WESTON NoRMAIv Cei.I< 477 A. Basis of reference for current and voltage measurements 478 1. Weston normal cells 478 2. Resistances 478 B. Meaning of the term " International volts" 478 C. Voltameters and materials 479 D. Tables of results 481 1. Corrections for acid present 482 2. Resultsof the porous pot and Smith's new-form voltameters. 485 3. Results of other forms of voltameters 492 4. Miscellaneous results 493 III. Speciai, Investigations 497 A. On the agreement of various forms 497 1 Comparative results 497 2 Possible explanations of observed differences 498 B. On the accuracy of the measurements. 501 C. Reliability of iodeosine as an indicator 502 D. Effect of acid 503 1 Effect on impure solutions 503 2 Nature of the action of acid 505 E. Influence of septa other than filter paper 508 1. Silk 508 2 Poroiis pots 510 3. Electrostenolysis 514 F. Purity of the silver deposit 516 1. Constancy of any error due to inclusions 516 2. Previous determinations of inclusions 517 3. Experiments on inclusions 519 (a) Detection of silver nitrate 519 (b) Estimation of included water and volatile material 52 (c) Estimation of organic material 522 (d) Titration of silver deposits 523 (e) Conclusion 523 475 476 Bulletin of the Bureau of Standards {Voi.io III. SpeciaIv Investigations—Continued. Page, G. Reversibility of the silver voltameter 523 H. -

Lecture Content
EMT 518: METHODS IN ENVIROMENTAL ANALYSIS III (2 UNITS) Lecturer: Professor O. Bamgbose SYNOPSIS Electro-analytical method: Potentiometry, Reference electrode – Calomel, Ag/Agcl, indicator electrodes – 1st, 2nd and 3rd order, Metal Electrodes, membrane electrodes – glass electrode, types of liquid junction potential, solid state electrode, potentiometric titration, end point location in potentiometric titration –visual, plot of E/V, plot of derivative curves 1st and 2nd electrogravimetry, fixed potential, constant current, constant cathode potential coulometry: constant current coulometry, coulometric titration. Voltammetry: classical polarography, Description of dropping mercury electrode, condition for polarographic determination, qualitative and quantitative analysis conductance methods: description of limiting ionic conductance, conductance cell, conductomertic titration. Thermal methods: Thermogravimetry, differential thermal analysis (DTA) LECTURE CONTENT POTENTIOMETRY Is a measurement of a given chemical species in an equilibrium system by the use of an electrode, while potentiometric titration is the technique that is used for following the changes in the concentration of chemical species as function of added titrant using an electrode. In both cases a cell is needed and a cell consists of the following: (1) Reference electrode (2) Liquid junction (3) Analyte solution (4) indicator electrode. It is also possible to have a cell without liquid junction. REFERENCE ELECTRODE. In carrying out a potentiometric determination the half cell potential of one electrode must be known which should be constant, reproducible and completely insensitive to the reference electrode and must be fully polarised throughout the duration of the measurement i.e the potential of the reference electrode does not change through the whole measurement. A classical example of reference electrode is the calomel electrode. -

Recommendation for Use Hofmann Voltameter for Educational Purposes
Glaswarenfabrik Tel. +49 (0) 9779 808 0 Karl Hecht Fax +49 (0) 9779 808 88 GmbH & Co KG Email: [email protected] Stettener Straße 22-24 Internet: www.assistent.eu 97647 Sondheim/Rhön VAT No. DE132198535 Germany Recommendation for use Hofmann voltameter for educational purposes Product number: 44285010 and 44286010 A water decomposition apparatus is used for the demonstration of electrolysis, i. e. for the decomposition of water into hydrogen and oxygen by means of electric current. The apparatus is consisting of a glass tube with pear-shaped funnel and two lateral measuring tubes 60 ml : 0.2 ml with glass stopcocks. There are two versions available: Product number 44285010 Spare parts Glass part No. 44287010 Metal tripod 2 Clamps No. 40870010 2 Platinum electrodes No. 44288010 Product number 44286010 Spare parts Glass part No. 44287010 Metal tripod 2 Clamps No. 40870010 2 Carbon electrodes No. 44289010 Experimental setup and test procedure Attach the voltameter with two clamps to the support. Insert the carbon or platinum electrodes into the measuring tubes. The stoppers have to be firmly in position in order to avoid leakage of liquid. For safety reasons place the apparatus into a glass tray. Pour in the electrolyte through the funnel while stopcocks are both open to let the air escape. Close the stopcocks as soon as the electrolyte has reached the level of stopcock bores. Connect the electrodes and the pole terminals at the tripod by means of two short connecting cables. Connect a DC voltage source and supply with electrical energy. The required direct voltage is about 10 V to 20 V at max. -

Physical Electrochemical Software Brochure
Redefining Electrochemical Measurement Physical Electrochemistry Software The Physical Electrochemistry Software is used with a to the limit. You can define the potentials as absolute Gamry Potentiostat to perform in-depth studies of the voltages or by their relationship to the Open-Circuit structure of the electrode interface and the mechanisms Potential. of electrochemical reactions. The software brings Cyclic Voltammetry and other recognized electrochemical The scan rate (mV/s) is determined by the interval between research techniques to the Gamry user. The Physical data points (sample period) and the Step Size (mV): Electrochemistry Software is a useful tool for Step Size() mV fundamental studies, sensor development, small-scale Scan Rate() mV s = energy storage devices, electrophysiology, etc. Sample Period() s The minimum sample period may be as low as 3.3 µs. The This software incorporates the following electrochemical maximum Scan Rate is a function of Step Size. For techniques: example, the maximum Scan Rate with a 2 mV step is 600 V/s. Higher steps provide faster scan rates, but at the ••• Cyclic Voltammetry expense of resolution. Step Sizes greater than 10 mV are ••• Linear Sweep Voltammetry likely to result in unsatisfactory data. ••• Chronoamperometry ••• Repeating Chronoamperometry The Physical Electrochemistry Software can save, and ••• MMuullttiipplleeMultiple-Multiple---StepStep Chronoamperometry display, up to 262,143 data points! The number of CV ••• ChronopotentChronopotentiometryiioommeettrryyiometry cycles that can be displayed is dependent upon the scan ••• Repeating Chronopotentiometry parameters. ••• Chronocoulometry Step Size() mV No. of Cycles =262,143 × ••• Controlled Potential Coulometry Voltage Span of theCV() mV Like most Gamry software, the Physical Electrochemistry Software and a Gamry Potentiostat use the Framework for data acquisition and the Echem Analyst for data analysis. -

Stationary Electrode Voltammetry and Chronoamperometry in an Alkali Metal Carbonate-Borate Melt
AN ABSTRACT OF THE THESIS OF DARRELL GEORGE PETCOFF for the Doctor of Philosophy (Name of student) (Degree) in Analytical Chemistry presented onC (O,/97 (Major) (Date) Title: STATIONARY ELECTRODE VOLTAMMETRY AND CHRONOAMPEROMETRY IN AN ALKALI METAL CARBONATE - BORATE. MFT T Abstract approved: Redacted for Privacy- Drir. reund The electrochemistry of the lithium-potassium-sodium carbonate-borate melt was explored by voltammetry and chrono- amperometry. In support of this, a controlled-potential polarograph and associated hardware was constructed.Several different types of reference electrodes were tried before choosing a porcelain mem- brane electrode containing a silver wire immersed in a silver sulfate melt.The special porcelain compounded was used also to construct a planar gold disk electrode.The theory of stationary electrode polarography was summarized and denormalized to provide an over- all view. A new approach to the theory of the cyclic background current was also advanced. A computer program was written to facilitate data processing.In addition to providing peak potentials, currents, and n-values, the program also resolves overlapping peaks and furnishes plots of both processed and unprocessed data. Rapid-scan voltammetry was employed to explore the electro- chemical behavior of Zn, Co, Fe, Tl, Sb, As, Ni, Sn, Cd, Te, Bi, Cr, Pb, Cu, and U in the carbonate-borate melt. Most substances gave reasonably well-defined peaks with characteristic peak potentials and n-values.Metal deposition was commonly accompanied by adsorp- tion prepeaks indicative of strong adsorption, and there was also evi- dence of a preceding chemical reaction for several elements, sug- gesting decomplexation before reduction. -

COULOMETRY for the DETERMINATION of URANIUM and PLUTONIUM: PAST and PRESENT by M.K
BARC/2012/E/001 BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division 2012 BARC/2012/E/001 GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division BHABHA ATOMIC RESEARCH CENTRE MUMBAI, INDIA 2012 BARC/2012/E/001 BIBLIOGRAPHIC DESCRIPTION SHEET FOR TECHNICAL REPORT (as per IS : 9400 - 1980) 01 Security classification : Unclassified 02 Distribution : External 03 Report status : New 04 Series : BARC External 05 Report type : Technical Report 06 Report No. : BARC/2012/E/001 07 Part No. or Volume No. : 08 Contract No. : 10 Title and subtitle : Coulometry for the determination of uranium and plutonium: past and present 11 Collation : 34 p., 2 figs., 7 tabs. 13 Project No. : 20 Personal author(s) : M.K. Sharma; J.V. Kamat; A.S. Ambolikar; J.S. Pillai; S.K. Aggarwal 21 Affiliation of author(s) : Fuel Chemistry Division, Bhabha Atomic Research Centre, Mumbai 22 Corporate author(s) : Bhabha Atomic Research Centre, Mumbai - 400 085 23 Originating unit : Fuel Chemistry Division, BARC, Mumbai 24 Sponsor(s) Name : Department of Atomic Energy Type : Government Contd... BARC/2012/E/001 30 Date of submission : December 2011 31 Publication/Issue date : January 2012 40 Publisher/Distributor : Head, Scientific Information Resource Division, Bhabha Atomic Research Centre, Mumbai 42 Form of distribution : Hard copy 50 Language of text : English 51 Language of summary : English, Hindi 52 No. -

Plutonium Analysis from Controlled-Potential Coulometry for the Certification of the MP3 Standard Material
P5_14 Plutonium analysis from controlled-potential coulometry for the certification of the MP3 standard material. A. Ruas, V. Dalier, J. Pivato CEA-Marcoule BP 17171 30207 Bagnols-sur-Cèze Cedex, France [email protected] Abstract – For contributing to the certification of the new metal plutonium reference material (MP3), controlled-potential coulometry (CPC) has many advantages: it is a high accuracy absolute chemical analysis technique. Many studies are now conducted on plutonium solutions, to improve the operating conditions and the current apparatus, for mass determination with a precision of 0.1%. The different experimental preliminary results are discussed and the apparatus described. The coulometry cell assembly comprises a motor connected to a stirrer designed to prevent splashing, an inlet tube for inert gas, three electrodes, and a thermocouple for measuring the temperature. The measuring system includes a potentiostat, a CPU, a calibrated current generator, a temperature indicator and a voltmeter, all maintained at a constant temperature. Current integration is made by electronic components, introduced in the potentiostat and the CPU. + − + INTRODUCTION Pu 4 + e ↔ Pu 3 (1) Experimental electrolysis is performed using a Coulometry is an assay method in which the metal electrode with a large surface area quantity of the element analyzed is determined (working electrode) immersed in the test by measuring a quantity of electricity; under solution. The quantity of electricity used for the certain conditions it is capable of providing a conversion is related to the quantity matter in very accurate determination of the plutonium solution by Faraday’s law of electrolysis: mass concentration. The advantage of this M method is that it is absolute and uses only small m = ⋅Q (2) masses of material. -

School of Engineering and Science to My Beloved Parents Abstract
Hybrid Organic-Inorganic Polyoxometalates Functionalized by Diorganotin Groups by Firasat Hussain A thesis submitted in partial ful¯llment of the requirements for the degree of Doctor of Philosophy Approved, Thesis Committee: Prof. Ulrich Kortz (Mentor), IUB Prof. Ryan M Richards, IUB Dr. Michael H Dickman, IUB Prof. Michael T Pope Georgetown University, U.S.A. Prof. Emmanuel Cadot Universit¶ede Versailles, France Date of defense: 19 May 2006 School of Engineering and Science To my beloved parents Abstract Polyoxometalates (POMs) are a well-known class of inorganic metal-oxygen clusters with an unmatched structural variety combined with a multitude of properties. The search for novel POMs is predominantly driven by exciting catalytic, medicinal, material science and bioscience applications. However, the mechanism of action of most polyoxoanions is not selective towards a speci¯c target. In order to improve selectivity it appears highly desirable to attach organic functionalities covalently to the surface of polyoxoanions. The hydrolytic stability of the Sn-C bond enables the synthesis of a novel class of polyoxoanions via attachment of organometallic functionalities based on Sn(IV) to the surface of lacunary polyoxoanion precursors. III III By reacting (CH3)2SnCl2 with Na9(®-XW9O33) (X = As , Sb ) in aqueous acidic medium leads to the formation of 2-D solid-state structures with inorganic and organic surface, which are rare examples of discrete polyoxoanions. (CsNa4f(Sn(CH3)2)3O(H2O)4 (¯-AsW9O33)g¢5H2O)1 (CsNa-1) and the isostructural (CsNa4[(Sn(CH3)2)3O(H2O)4( ¯-SbW9O33)]¢5H2O)1 (CsNa-2)It has been synthesized and characterized by multinu- clear NMR spectroscopy, FTIR spectroscopy and elemental analysis. -
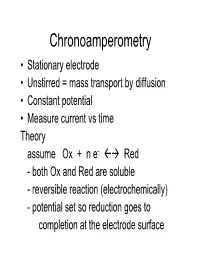
Chronoamperometry
Chronoamperometry • Stationary electrode • Unstirred = mass transport by diffusion • Constant potential • Measure current vs time Theory assume Ox + n e- Red - both Ox and Red are soluble - reversible reaction (electrochemically) - potential set so reduction goes to completion at the electrode surface Components of output signal in Chronoamperometry IFar decreases because Ox used up at electrode surface and Ox is only replenished by diffusion Faradaic current (IFar) follows Cottrell equation I Capacitive current (Icap) (current) decays exponentially for a constant applied potential t (time) Icap is high as electrode capacitive layer charges up, then drops off Processes perturbing system can cause data to differ from Cottrell Equation 1) Capacitive Current – charging current is exponential as shown -kt Icap = e Note: Capacitive current decreases more rapidly than Faradaic current so at longer times the ratio IFar/Icap is larger 2) Occurrence of coupled chemical reactions e.g. Ox + n e- Red 2 Red A A + n e- B Affects the shape of the current-time curve Chronoamperometry Applications • Can measure concentration by measuring I vs conc. at any fixed time • Can analyze the shape of the current-time curve in order to study coupled chemical reactions • There are better ways to do both of these with more modern techniques • Chronoamperometry is important because it is a fundamental method on which other techniques are based Chronopotentiometry • Stationary electrode • Unstirred = mass transport by diffusion • Constant current applied between -

The High-Precision Thermometric and Coulometric Assay of Primary Standard 4-Aminopyridine William Charles Hoyle Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1973 The high-precision thermometric and coulometric assay of primary standard 4-aminopyridine William Charles Hoyle Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons Recommended Citation Hoyle, William Charles, "The high-precision thermometric and coulometric assay of primary standard 4-aminopyridine " (1973). Retrospective Theses and Dissertations. 5017. https://lib.dr.iastate.edu/rtd/5017 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

INDIAN INSTITUTE of TECHNOLOGY KANPUR Department of Civil Engineering Environmental Engineering and Management Programme Kanpur-208016, INDIA
INDIAN INSTITUTE OF TECHNOLOGY KANPUR Department of Civil Engineering Environmental Engineering and Management Programme Kanpur-208016, INDIA Dr. Vinod Tare Professor Tel : +91 512 259 7792 (O), Fax : +91 512 259 7395 (O); E-mail : [email protected] Enquiry Letter No. CE/EEM/2013/NC/VA/10 Date: Aug 22, 2013 Last Date: September 9, 2013 Letter Requesting Quotation for Voltameter Quotation is sought for a Voltameter with minimum specifications given in the list below. The quotation has to be submitted in two separately sealed envelopes, one containing the technical specifications and marked as “Technical Quote” on the envelope and the other containing the price and marked as the “Price Quote” on the envelope. Please apply the discounts admissible to the educational institutes such as IIT Kanpur and also note that IIT Kanpur is exempted from paying duties for imported items. Minimum Specification Required: • Voltameter: determination of transition metals like copper, tin ,vanadium , mercury and other metals based on Voltammetry principle with wide-band low-noise amplifier, potentiostat and galvanostat. Voltage Range: ± 5 V, Output Range: ± 12 V, Current Range: ± 80 mA with measurement resolution of 10nA to 10mA, sweep rate of 1mV/s to 3V/s and/or 35V/s • Electrode System: multimode SMDE, HMDE and DME, choice of multiple drop sizes, should minimize mercury requirement. Rotating disc electrode facility and various solid-state electrodes like Gold, Silver, Glassy Carbon, Platinum and Graphite. • Instrument Control and Analysis: Computer and Software must be provided for fully computer controlled operation, data acquisition and quantitative analysis. Software must be able to analyse by following voltametric techniques: Direct Current (DC), Sampled DC, Differential Pulse (DP), Square-wave (SQW), Alternating Current (AC) 1st and 2nd harmonic, phase selective, Cyclic Voltammetry (CV), Potentometric Stripping Analysis (PSA), Normal Pulse (NP) and Constant Current Potentiometric Stripping Analysis (CCPSA).