Plutonium Analysis from Controlled-Potential Coulometry for the Certification of the MP3 Standard Material
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture Content
EMT 518: METHODS IN ENVIROMENTAL ANALYSIS III (2 UNITS) Lecturer: Professor O. Bamgbose SYNOPSIS Electro-analytical method: Potentiometry, Reference electrode – Calomel, Ag/Agcl, indicator electrodes – 1st, 2nd and 3rd order, Metal Electrodes, membrane electrodes – glass electrode, types of liquid junction potential, solid state electrode, potentiometric titration, end point location in potentiometric titration –visual, plot of E/V, plot of derivative curves 1st and 2nd electrogravimetry, fixed potential, constant current, constant cathode potential coulometry: constant current coulometry, coulometric titration. Voltammetry: classical polarography, Description of dropping mercury electrode, condition for polarographic determination, qualitative and quantitative analysis conductance methods: description of limiting ionic conductance, conductance cell, conductomertic titration. Thermal methods: Thermogravimetry, differential thermal analysis (DTA) LECTURE CONTENT POTENTIOMETRY Is a measurement of a given chemical species in an equilibrium system by the use of an electrode, while potentiometric titration is the technique that is used for following the changes in the concentration of chemical species as function of added titrant using an electrode. In both cases a cell is needed and a cell consists of the following: (1) Reference electrode (2) Liquid junction (3) Analyte solution (4) indicator electrode. It is also possible to have a cell without liquid junction. REFERENCE ELECTRODE. In carrying out a potentiometric determination the half cell potential of one electrode must be known which should be constant, reproducible and completely insensitive to the reference electrode and must be fully polarised throughout the duration of the measurement i.e the potential of the reference electrode does not change through the whole measurement. A classical example of reference electrode is the calomel electrode. -

Physical Electrochemical Software Brochure
Redefining Electrochemical Measurement Physical Electrochemistry Software The Physical Electrochemistry Software is used with a to the limit. You can define the potentials as absolute Gamry Potentiostat to perform in-depth studies of the voltages or by their relationship to the Open-Circuit structure of the electrode interface and the mechanisms Potential. of electrochemical reactions. The software brings Cyclic Voltammetry and other recognized electrochemical The scan rate (mV/s) is determined by the interval between research techniques to the Gamry user. The Physical data points (sample period) and the Step Size (mV): Electrochemistry Software is a useful tool for Step Size() mV fundamental studies, sensor development, small-scale Scan Rate() mV s = energy storage devices, electrophysiology, etc. Sample Period() s The minimum sample period may be as low as 3.3 µs. The This software incorporates the following electrochemical maximum Scan Rate is a function of Step Size. For techniques: example, the maximum Scan Rate with a 2 mV step is 600 V/s. Higher steps provide faster scan rates, but at the ••• Cyclic Voltammetry expense of resolution. Step Sizes greater than 10 mV are ••• Linear Sweep Voltammetry likely to result in unsatisfactory data. ••• Chronoamperometry ••• Repeating Chronoamperometry The Physical Electrochemistry Software can save, and ••• MMuullttiipplleeMultiple-Multiple---StepStep Chronoamperometry display, up to 262,143 data points! The number of CV ••• ChronopotentChronopotentiometryiioommeettrryyiometry cycles that can be displayed is dependent upon the scan ••• Repeating Chronopotentiometry parameters. ••• Chronocoulometry Step Size() mV No. of Cycles =262,143 × ••• Controlled Potential Coulometry Voltage Span of theCV() mV Like most Gamry software, the Physical Electrochemistry Software and a Gamry Potentiostat use the Framework for data acquisition and the Echem Analyst for data analysis. -

Stationary Electrode Voltammetry and Chronoamperometry in an Alkali Metal Carbonate-Borate Melt
AN ABSTRACT OF THE THESIS OF DARRELL GEORGE PETCOFF for the Doctor of Philosophy (Name of student) (Degree) in Analytical Chemistry presented onC (O,/97 (Major) (Date) Title: STATIONARY ELECTRODE VOLTAMMETRY AND CHRONOAMPEROMETRY IN AN ALKALI METAL CARBONATE - BORATE. MFT T Abstract approved: Redacted for Privacy- Drir. reund The electrochemistry of the lithium-potassium-sodium carbonate-borate melt was explored by voltammetry and chrono- amperometry. In support of this, a controlled-potential polarograph and associated hardware was constructed.Several different types of reference electrodes were tried before choosing a porcelain mem- brane electrode containing a silver wire immersed in a silver sulfate melt.The special porcelain compounded was used also to construct a planar gold disk electrode.The theory of stationary electrode polarography was summarized and denormalized to provide an over- all view. A new approach to the theory of the cyclic background current was also advanced. A computer program was written to facilitate data processing.In addition to providing peak potentials, currents, and n-values, the program also resolves overlapping peaks and furnishes plots of both processed and unprocessed data. Rapid-scan voltammetry was employed to explore the electro- chemical behavior of Zn, Co, Fe, Tl, Sb, As, Ni, Sn, Cd, Te, Bi, Cr, Pb, Cu, and U in the carbonate-borate melt. Most substances gave reasonably well-defined peaks with characteristic peak potentials and n-values.Metal deposition was commonly accompanied by adsorp- tion prepeaks indicative of strong adsorption, and there was also evi- dence of a preceding chemical reaction for several elements, sug- gesting decomplexation before reduction. -

COULOMETRY for the DETERMINATION of URANIUM and PLUTONIUM: PAST and PRESENT by M.K
BARC/2012/E/001 BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division 2012 BARC/2012/E/001 GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION BARC/2012/E/001 COULOMETRY FOR THE DETERMINATION OF URANIUM AND PLUTONIUM: PAST AND PRESENT by M.K. Sharma, J.V. Kamat, A.S. Ambolikar, J.S. Pillai and S.K. Aggarwal Fuel Chemistry Division BHABHA ATOMIC RESEARCH CENTRE MUMBAI, INDIA 2012 BARC/2012/E/001 BIBLIOGRAPHIC DESCRIPTION SHEET FOR TECHNICAL REPORT (as per IS : 9400 - 1980) 01 Security classification : Unclassified 02 Distribution : External 03 Report status : New 04 Series : BARC External 05 Report type : Technical Report 06 Report No. : BARC/2012/E/001 07 Part No. or Volume No. : 08 Contract No. : 10 Title and subtitle : Coulometry for the determination of uranium and plutonium: past and present 11 Collation : 34 p., 2 figs., 7 tabs. 13 Project No. : 20 Personal author(s) : M.K. Sharma; J.V. Kamat; A.S. Ambolikar; J.S. Pillai; S.K. Aggarwal 21 Affiliation of author(s) : Fuel Chemistry Division, Bhabha Atomic Research Centre, Mumbai 22 Corporate author(s) : Bhabha Atomic Research Centre, Mumbai - 400 085 23 Originating unit : Fuel Chemistry Division, BARC, Mumbai 24 Sponsor(s) Name : Department of Atomic Energy Type : Government Contd... BARC/2012/E/001 30 Date of submission : December 2011 31 Publication/Issue date : January 2012 40 Publisher/Distributor : Head, Scientific Information Resource Division, Bhabha Atomic Research Centre, Mumbai 42 Form of distribution : Hard copy 50 Language of text : English 51 Language of summary : English, Hindi 52 No. -

School of Engineering and Science to My Beloved Parents Abstract
Hybrid Organic-Inorganic Polyoxometalates Functionalized by Diorganotin Groups by Firasat Hussain A thesis submitted in partial ful¯llment of the requirements for the degree of Doctor of Philosophy Approved, Thesis Committee: Prof. Ulrich Kortz (Mentor), IUB Prof. Ryan M Richards, IUB Dr. Michael H Dickman, IUB Prof. Michael T Pope Georgetown University, U.S.A. Prof. Emmanuel Cadot Universit¶ede Versailles, France Date of defense: 19 May 2006 School of Engineering and Science To my beloved parents Abstract Polyoxometalates (POMs) are a well-known class of inorganic metal-oxygen clusters with an unmatched structural variety combined with a multitude of properties. The search for novel POMs is predominantly driven by exciting catalytic, medicinal, material science and bioscience applications. However, the mechanism of action of most polyoxoanions is not selective towards a speci¯c target. In order to improve selectivity it appears highly desirable to attach organic functionalities covalently to the surface of polyoxoanions. The hydrolytic stability of the Sn-C bond enables the synthesis of a novel class of polyoxoanions via attachment of organometallic functionalities based on Sn(IV) to the surface of lacunary polyoxoanion precursors. III III By reacting (CH3)2SnCl2 with Na9(®-XW9O33) (X = As , Sb ) in aqueous acidic medium leads to the formation of 2-D solid-state structures with inorganic and organic surface, which are rare examples of discrete polyoxoanions. (CsNa4f(Sn(CH3)2)3O(H2O)4 (¯-AsW9O33)g¢5H2O)1 (CsNa-1) and the isostructural (CsNa4[(Sn(CH3)2)3O(H2O)4( ¯-SbW9O33)]¢5H2O)1 (CsNa-2)It has been synthesized and characterized by multinu- clear NMR spectroscopy, FTIR spectroscopy and elemental analysis. -
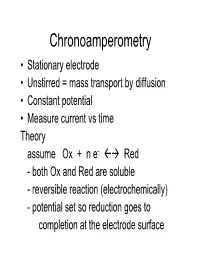
Chronoamperometry
Chronoamperometry • Stationary electrode • Unstirred = mass transport by diffusion • Constant potential • Measure current vs time Theory assume Ox + n e- Red - both Ox and Red are soluble - reversible reaction (electrochemically) - potential set so reduction goes to completion at the electrode surface Components of output signal in Chronoamperometry IFar decreases because Ox used up at electrode surface and Ox is only replenished by diffusion Faradaic current (IFar) follows Cottrell equation I Capacitive current (Icap) (current) decays exponentially for a constant applied potential t (time) Icap is high as electrode capacitive layer charges up, then drops off Processes perturbing system can cause data to differ from Cottrell Equation 1) Capacitive Current – charging current is exponential as shown -kt Icap = e Note: Capacitive current decreases more rapidly than Faradaic current so at longer times the ratio IFar/Icap is larger 2) Occurrence of coupled chemical reactions e.g. Ox + n e- Red 2 Red A A + n e- B Affects the shape of the current-time curve Chronoamperometry Applications • Can measure concentration by measuring I vs conc. at any fixed time • Can analyze the shape of the current-time curve in order to study coupled chemical reactions • There are better ways to do both of these with more modern techniques • Chronoamperometry is important because it is a fundamental method on which other techniques are based Chronopotentiometry • Stationary electrode • Unstirred = mass transport by diffusion • Constant current applied between -

High Speed Controlled Potential Coulometry
c1CYCLIC CHELONO, DIFPU- c2SOLVE GENERATED EQUA- 903 FORMAT (5HRR =, F10.5, SION CONTROLL, PLANE TION BEGIN AT 96 READ 8HFRACT =, F10.5) ELECTRODE, READ IN K IN NOSIG FOR ACCURACY GO TO 920 NOSIG RR FRACT, TWO 96 IF(M- 1)300,100,102 300 PRINT905 SOLUBLE ElPECIES 100 Z=Y 905 FORRSAT (2X,5HEItROR) READ 900,K,NOSlG, RR, M=M+l 920 STOP FRACT 102 IF (Z) 98,200,99 EXD DIMENSION X (100),T (1 00) , 98 IF (Y) 71,200,73 END R(100) 99 IF (Y) 73,200,71 C GENERATION OF EQUA- 71 T(N) = T(N) + 10.0 **(-LA) LITERATURE CITED TIONS GO TO 10 (1) Alden, J. R., Chambers, J. Q., Adams, DO200N = 1,K 73 T(N) = T(K) - 10.0 **(-LA) R. N., J. Electroanal. Chem. 5, 152 T(N) = 0.0 LA=LA+I (1963). M=l 199 IF (NOSIG - LA) 300,200,71 (2) Bard, A. J., ANAL. CHEM. 33, 11 (1961). LA = 0 200 CONTIXUE (3) Churchill, R. V., “Operational Mathe- 10 DO 80 I = 1,N c3EQUATION SOLVED PRINT matics,” p. 39, McGraw-Hill, New York, SUM = 0.0 ANSWER 1958. DO 60 J = I,N DO201 J = 1,K,2 (4) Galus, Z., Lee, H. Y., Adams, R. N., = 201 R(J) = T(J)/T(J 1) J. Electroanal. Chem. 5, 17 (1963). 60 SUM SUM -- T(J) + (5) Murra,y, R. W., Reilley, C. N., Ibid., X(1) = SQRTF(SUM) PRINT 903, RR, FRACT 3, 182 (1962). 80 CONTIXUE PRINT 901 (6) Piette, L. -
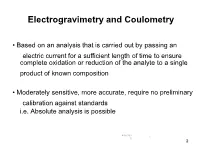
Electrogravimetry and Coulometry
Electrogravimetry and Coulometry • Based on an analysis that is carried out by passing an electric current for a sufficient length of time to ensure complete oxidation or reduction of the analyte to a single product of known composition • Moderately sensitive, more accurate, require no preliminary calibration against standards i.e. Absolute analysis is possible 4/16/202 1 0 2 Electrogravimetry - The product is weighed as a deposit on one of the electrodes (the working electrode) • Constant Applied Electrode Potential • Controled Working Electrode Potential Coulometry - • The quantity of electrical charge needed to complete the electrolysis is measured • Types of coulometric methods Controlled- potential coulometry Coulometric titrimetry 4/16/202 2 0 3 Electrogravimetric Methods • Involve deposition of the desired metallic element upon a previously weighed cathode, followed by subsequent reweighing of the electrode plus deposit to obtain by difference the quantity of the metal • Cd, Cu, Ni, Ag, Sn, Zn can be determined in this manner • Few substances may be oxidized at a Pt anode to form an insoluble and adherent precipitate suitable for gravimetric measurement 3 e.g. oxidation of lead(II) to lead dio4/1xi6/20d2 e in HNO acid 3 0 4 • Certain analytical separations can be accomplished Easily reducible metallic ions are deposited onto a mercury pool cathode Difficult-to-reduce cations remain in solution Al, V, Ti, W and the alkali and alkaline earth metals may be separated from Fe, Ag, Cu, Cd, Co, and Ni by deposition of the latter group of elements onto mercury 4/16/202 4 0 5 Constant applied potential (no control of the working electrode potential) Electrogravimetric methods Controlled working electrode potential 4/16/202 5 0 6 Constant applied potential electrogravimetry • Potential applied across the cell is maintained at a constant level throughout the electrolysis • Need a simple and inexpensive equipment • Require little operator attention • Apparatus consists of I). -

Simultaneous Coulcmetric Analtsis Dissertation
SIMULTANEOUS COULCMETRIC ANALTSIS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By RICHARD DONALD McIVER, B«A. The Ohio State University 195k Approved by Adviser ACKNGWLED GMENTS I wish to express ny sincere appreciation to Dr® William MacNevin, a most competent adviser and friend, to the Proctor and Gamble Comparer and the Al lied Chemical Company both of which awarded me fellow ships, and to u$r wife who was both an inspiration and help during ray graduate studies® I also wish to thank Dr. Bertsil Baker for his assistance and suggestions and to the Department of Chemistry of The Ohio State University in which I served two years as a Graduate Assistant. i A. 4 8 2 7 2 TABLE OF CONTENTS page THE PROBLEM.............................................. 1 INI R QDUCTI O N ............................................ 2 The Faraday and Electrolysis.......................... 2 Instruments for Measuring the Number of Coulombs * . • • 2 Electrolysis at Constant Current ............... 3 Electrolysis at Controlled Potential ......... .... 5 i Devices for Controlling Electrode Potential ....... 6 Coulometric A n a l y s i s ................................. 7 Constant Current Coulometry ...................... * . 8 Controlled Electrode Potential Coulometry.............. 10 Coulometry Applied to Indirect Analysis ......... 11 Indirect Analysis and Simultaneous Equations........... 12 Propagation of Errors in Simultaneous Equations ......... 1U Applications of Coulometry to Indirect Analysis ..... 18 EXPERIMENTAL........................................... 20 General Apparatus and Materials ........ 20 Apparatus for the Automatic Control of Electrode Potential •• ........................... 20 Balance and Weights ......... ....... ... 2h Reagents ......... 2U Saturated Calomel Electrode ............. 2$ Barometer......... 25 Hydrogen-Ctxygen Coulometer...................... 25 ii Table of Contents (continued) Page Investigations with the Rydrogen-Qxygen Coulometer . -

Anodic Stripping Voltammetry Introduction
Department of Environmental Science M.Sc. (Environmental Science) 2nd Semester MSESC 2006E04 Analytical Methods Course Instructor: Dr. Rajesh Kumar Ranjan ANODIC STRIPPING VOLTAMMETRY INTRODUCTION • Stripping analysis is an analytical technique that involves (i) pre concentration of a metal phase onto a solid electrode surface or into Hg (liquid) at negative potentials and (ii) selective oxidation of each metal phase species during an anodic potential sweep. • Excellent technique for trace metal ion analysis. • Pre concentration leads to low detection limits. • Very sensitive and reproducible method for trace metal ion analysis. • Useful for the analysis of very dilute solutions, 10-10 M. • Concentration limits of detection for many metals are in the low ppb to ppt. • Approximately 12-15 metal ions can be analysed for by this method. • The stripping peak currents and peak widths are a function of the size, coverage and distribution of the metal phase on the electrode surface (Hg or alternate) HISTORY • Electroanalytical techniques- Major families of instrumental method- applicable to qualitative & quantitative analysis- have 3 branches: I. Potentiometry II. Voltametry III.Coulometry • Voltammetry started with the development of polarography- Heyrovsky 1920. • ASV first used by Zbinden in 1931. • 1950s – mercury electrode become common. • 1960s – MFEs & HMDEs was developed. (Ellis, 1973) PRINCIPLE • Applicable to metal ion detection at an electrode. • Accumulated by potentiostatic deposition onto a small-volume Hg electrode. • Cathodic deposition at a controlled time and potential. • Stripping analysis. FIG 1: Principle of ASV. Values shown are typical one ; potential and Ep are typical of Cu 2+ analysis. (a) preelctrolysisi at E; stirred solution. (b) rest period; stirrer off. -

A Review of Neurotransmitters Sensing Methods for Neuro-Engineering Research
applied sciences Review A Review of Neurotransmitters Sensing Methods for Neuro-Engineering Research Shimwe Dominique Niyonambaza 1,2, Praveen Kumar 1, Paul Xing 3, Jessy Mathault 1, Paul De Koninck 1,4, Elodie Boisselier 2,5 , Mounir Boukadoum 6 and Amine Miled 1,* 1 LABioTRON Bioeng. Research Laboratory, ECE Dept. Université Laval, Québec City, QC G1V 0A6, Canada; [email protected] (S.D.N.); [email protected] (P.K.); [email protected] (J.M.); [email protected] (P.D.K.) 2 CUO-Recherche, Hôpital du Saint-Sacrement, Québec City, QC G3K 1A3, Canada; [email protected] 3 Neurosciences Dept. University of Montreal, Montreal, QC H3C 3J7, Canada; [email protected] 4 CERVO Brain Research Center, Québec City, QC G1J 2G3, Canada 5 Ophthalmology Department, Faculty of Medicine, Université Laval, Québec City, QC G1V 0A6, Canada 6 CoFaMic, Université du Québec à Montréal, Montreal, QC H2L 2C4, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-(418)-656-2131 (ext. 8966) Received: 1 May 2019; Accepted: 11 October 2019; Published: 5 November 2019 Abstract: Neurotransmitters as electrochemical signaling molecules are essential for proper brain function and their dysfunction is involved in several mental disorders. Therefore, the accurate detection and monitoring of these substances are crucial in brain studies. Neurotransmitters are present in the nervous system at very low concentrations, and they mixed with many other biochemical molecules and minerals, thus making their selective detection and measurement difficult. Although numerous techniques to do so have been proposed in the literature, neurotransmitter monitoring in the brain is still a challenge and the subject of ongoing research. -

A Method for the Identification of the Products from Controlled
THACKER, JR., FRANKLIN AUBREY. A Method for the Identification of the Products from Controlled-Potential Coulometry of p-Nitrosophenol, p-Nitrophenol and p-(p-Hydroxyphenylazo)- benzenesulfonic Acid Sodium Salt. (1976) Directed by: Dr. Harvey B. Herman. Pp.44 The aim of this study was to identify the controlled- potential coulometry products after electrolytic reduction of p-nitrosophenol, p-nitrophenol, p-(p-hydroxyphenylazo)- benzenesulfonic acid sodium salt, Orange I, and Orange II. Polarography was performed on all individual compounds in two buffer solutions at pH 4.9 and 11.8 using the dropping mercury electrode polarographic circuit. The resulting polar- ograms were used to select the controlled working electrode potentials for coulometry. Coulometry was conducted with the individual compounds and each pH buffer solution in a stirred mercury pool coulometry cell. The potential was controlled and the number of electrons (n) involved in the reduction process was determined with the aid of an electrical circuit whose output voltage was propor- tional to n. The coulometric reaction solutions were evaporated and the product residues were treated with silylating reagent to form silyl derivatives. The silyl derivatives increased product volatilities so that identification by gas chromatography was possible. Gas chromatography was performed using a packed glass column. The silylated coulometric product was detected by flame ionization. v , As expected, the nitroso, nitro and azo compounds gave coulometric n values equal to 4, 6, and 4 respectively. Also, p-aminophenol was identified as the product after coulometry of p-nitrosophenol, p-nitrophenol, and p-(p-hydroxyphenylazo)- benzenesulfonic acid sodium salt. The coulometry products of Orange I and Orange II were not able to be identified by gas chromatography, apparently because of instability of the anticipated amino-naphthol compounds.