A Review of Endiandric Acid Analogues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Take Another Look
Take Contact Details Another SUNSHINE COAST REGIONAL COUNCIL Caloundra Customer Service Look..... 1 Omrah Avenue, Caloundra FRONT p: 07 5420 8200 e: [email protected] Maroochydore Customer Service 11-13 Ocean Street, Maroochydore p: 07 5475 8501 e: [email protected] Nambour Customer Service Cnr Currie & Bury Street, Nambour p: 07 5475 8501 e: [email protected] Tewantin Customer Service 9 Pelican Street, Tewantin p: 07 5449 5200 e: [email protected] YOUR LOCAL CONTACT Our Locals are Beauties HINTERLAND EDITION HINTERLAND EDITION 0 Local native plant guide 2 What you grow in your garden can have major impact, Introduction 3 for better or worse, on the biodiversity of the Sunshine Native plants 4 - 41 Coast. Growing a variety of native plants on your property can help to attract a wide range of beautiful Wildlife Gardening 20 - 21 native birds and animals. Native plants provide food and Introduction Conservation Partnerships 31 shelter for wildlife, help to conserve local species and Table of Contents Table Environmental weeds 42 - 73 enable birds and animals to move through the landscape. Method of removal 43 Choosing species which flower and fruit in different Succulent plants and cacti 62 seasons, produce different types of fruit and provide Water weeds 70 - 71 roost or shelter sites for birds, frogs and lizards can greatly increase your garden’s real estate value for native References and further reading 74 fauna. You and your family will benefit from the natural pest control, life and colour that these residents and PLANT TYPE ENVIRONMENTAL BENEFITS visitors provide – free of charge! Habitat for native frogs Tall Palm/Treefern Local native plants also improve our quality of life in Attracts native insects other ways. -

IFE and Wlldll ITAT of AMERICAN SA VIRONMENT and ECOLO
IFE AND WlLDLl ITAT OF AMERICAN SA VIRONMENT AND ECOLO By A. Binion Amerson, Jr., W. Arthur Whistler, and Terry D. Schwaner Environment Consultants, Inc., Dallas, Texas Edited by Richard C. Banks U.S. Fish and Wildlife Service Washington, D.C. UNITED STATES DEPARTMENT OF T E INTERIOR FISH AND WILDLIFE SERVICE Washington, D.C. e 1982 Foreword A survey of the status of the wildlife and wildlife habitat of American Samoa. an unincorporated Territory of the United States. was recommended by administrative officials of the U.S. Fish and Wildlife Service (FWS) in the early 1970s .Environ- ment Consultants. Inc . (ECI). based in Dallas. Texas. was selected to conduct a 2-year survey with A .Binion Amerson. Jr., as Principal Investigator . The contract was administered through the Division of Federal Aid in FWS Region I. Portland. Oregon . The primary objectives of the survey were (1) to define the major ecosystems and to inventory their physical components. vegetation. and wildlife constituents; (2) to prepare maps of these ecosystems; (3) to identify any threatened or endangered species of wildlife; and (4) to recommend wildlife management opportunities and needs . The report of the survey was to be in two parts . The first was to be a non-technical account suitable for wide general distribution; the second was to include the technical aspects of the data and data gathering. with accounts of the wildlife species . This volume represents the first part of ECI's report . The final report submitted by ECI contained more than 1. 200 pages. 200 figures. and 110 tables. many of thelatter several pages long . -

Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae)
Biomolecules 2015, 5, 910-942; doi:10.3390/biom5020910 OPEN ACCESS biomolecules ISSN 2218-273X www.mdpi.com/journal/biomolecules/ Review Endiandric Acid Derivatives and Other Constituents of Plants from the Genera Beilschmiedia and Endiandra (Lauraceae) Bruno Ndjakou Lenta 1,2,*, Jean Rodolphe Chouna 3, Pepin Alango Nkeng-Efouet 3 and Norbert Sewald 2 1 Department of Chemistry, Higher Teacher Training College, University of Yaoundé 1, P.O. Box 47, Yaoundé, Cameroon 2 Organic and Bioorganic Chemistry, Chemistry Department, Bielefeld University, P.O. Box 100131, 33501 Bielefeld, Germany; E-Mail: [email protected] 3 Department of Chemistry, University of Dschang, P.O. Box 67, Dschang, Cameroon; E-Mails:[email protected] (J.R.C.); [email protected] (P.A.N.-E.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +2376-7509-7561. Academic Editor: Jürg Bähler Received: 3 March 2015 / Accepted: 6 May 2015 / Published: 14 May 2015 Abstract: Plants of the Lauraceae family are widely used in traditional medicine and are sources of various classes of secondary metabolites. Two genera of this family, Beilschmiedia and Endiandra, have been the subject of numerous investigations over the past decades because of their application in traditional medicine. They are the only source of bioactive endiandric acid derivatives. Noteworthy is that their biosynthesis contains two consecutive non-enzymatic electrocyclic reactions. Several interesting biological activities for this specific class of secondary metabolites and other constituents of the two genera have been reported, including antimicrobial, enzymes inhibitory and cytotoxic properties. This review compiles information on the structures of the compounds described between January 1960 and March 2015, their biological activities and information on endiandric acid biosynthesis, with 104 references being cited. -
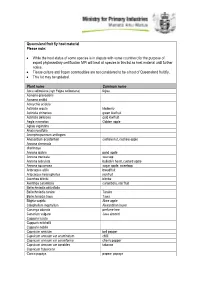
MPI Queensland Fruit Fly Host Material List
Queensland fruit fly host material Please note: While the host status of some species is in dispute with some countries; for the purpose of export phytosanitary certification MPI will treat all species in this list as host material until further notice. Tissue culture and frozen commodities are not considered to be a host of Queensland fruit fly. This list may be updated. Plant name Common name Acca sellowiana (syn Feijoa sellowiana) feijoa Acmena graveolens Acmena smithii Acroychia acidula Actinidia arguta kiwiberry Actinidia chinensis green kiwifruit Actinidia deliciosa gold kiwifruit Aegle marmelos Golden apple Aglaia sapindina Alyxia ruscifolia Amorphospermum antilogum Anacardium occidentale cashew nut, cashew apple Annona cherimola cherimoya Annona glabra pond apple Annona muricata soursop Annona reticulata bullock's heart, custard apple Annona squamosa sugar apple, sweetsop Artocarpus altilis breadfruit Artocarpus heterophyllus jackfruit Averrhoa bilimbi blimbe Averrhoa carambola carambola, star fruit Beilschmiedia obtusifolia Beilschmiedia taraire Taraire Beilschmiedia tawa Tawa Blighia sapida Akee apple Calophyllum inophyllum Alexandrian laurel Cananga odorata perfume tree Canarium vulgare Java almond Capparis lucida Capparis mitchellii Capparis nobilis Capsicum annuum bell pepper Capsicum annuum var acuminatum chilli Capsicum annuum var cerasiforme cherry pepper Capsicum annuum var conoides tabasco Capsicum frutescens Carica papaya papaw, papaya Carica pentagona babaco Carissa ovata Casimiroa edulis white sapote Cassine australia Castanospora alphandii Chrysophyllum cainito caimito, star apple Cissus sp Citrullus lanatus Watermelon Citrus spp. Citrus aurantiifolia West indian lime Citrus aurantium Seville orange Citrus grandis (syn maxima) pummelo Citrus hystrix kaffir lime Citrus jambhiri rough lemon Citrus latifolia Tahatian lime Citrus limetta sweet lemon tree Citrus limon lemon Citrus maxima (syn grandis) pomelo, shaddock Citrus medica citron Citrus meyeri Meyer lemon Citrus reticulata mandarin Citrus reticulata var. -

Threatened Species of Wilsons and Coopers Creek
Listed below are species recorded from the project areas of Goonengerry Landcare and Wilsons Creek Huonbrook Landcare groups. Additional species are known from adjacent National Parks. E = Endangered V = Vulnerable BCA - Biodiversity Conservation Act 2016 EPBC - Environment Protection and Biodiversity Conservation Act 1999 Threatened Species of Wilsons and Coopers Creek SOS - Saving our Species Scientific name Common name TSC Act status EPBC Act status SOS stream Wilsons Creek and Coopers Creek are tributaries of the Wilsons River on the Far North Coast of New South Wales. Within the South East Queensland Bioregion, the native flora and fauna of PLANTS this region are among the most diverse in Australia. In the catchment areas of the Wilsons and Corokia whiteana Corokia V V Keep watch Coopers Creek 50 threatened species of flora and fauna can be found and 2 endangered Davidsonia johnsonii Smooth Davidson's Plum E E Site managed ecological communities. Desmodium acanthocladum Thorny Pea V V Site managed What is a threatened species? Diploglottis campbellii Small-leaved Tamarind E E Site managed Plants and animals are assessed on the threats that face them and the level to which they are at Doryanthes palmeri Giant Spear Lily V Keep watch risk of extinction. If the risk is high they are listed in legislation and conservation actions are Drynaria rigidula Basket Fern E Partnership developed for their protection. There are almost 1000 animal and plant species at risk of Elaeocarpus williamsianus Hairy Quandong E E Site managed extinction in NSW. Endiandra hayesii Rusty Rose Walnut V V Data deficient A species is considered threatened if: Endiandra muelleri subsp. -

Coolum Street Tree Strategy
Coolum Street tree strategy Description of plan area and land use Major opportunities and constraints Street tree planting strategies Coolum provides a casual seaside setting for urban residential living and The best street tree planting opportunities in the Coolum locality exist in the tourism. The plan area covers the established seaside suburbs of Coolum, form of planting nodes, where large trees or groups of trees can be readily Street tree plantings reinforce the casual beachside character of Point Arkwright, Yaroomba and Mount Coolum, the newer residential accommodated. the precinct and are considerate of existing scenic amenity and the developments of the Boardwalk and the Town of Seaside, and the Coolum Significant opportunity to plant succession trees in areas of foreshore that preservation of existing water views. Industrial Park west of the Sunshine Motorway. Covering 1880 hectares, the intersect with streets is evident. Planting trees in areas of foreshore where landscape and extent of tree cover varies considerably, yet a relaxed urban Streetscapes predominately exhibit mixed native species plantings. no water views exist is a key priority. coastal character is consistent throughout the locality. Gateways and node plantings on Coolum–Yandina Road and the Additional street tree planting opportunities include the potential to infill Esplanade enhance the sense of arrival to the precinct. Trees and landscape character major avenues with new street trees, build canopy on parkland perimeters (fronting local streets) and provide shade to numerous pedestrian routes Succession trees are planted wherever there are no water views and The Coolum plan area contains many significant natural features including and pathways. -

Severe Tropical Cyclone Larry
The effects of Severe Tropical Cyclone Larry on rainforest vegetation and understorey microclimate adjacent to powerlines, highways and streams in the Wet Tropics World Heritage Area Catherine L. Pohlman and Miriam Goosem School of Earth and Environmental Sciences, James Cook University Supported by the Australian Government’s Marine and Tropical Sciences Research Facility Project 4.9.3 Impacts of urbanisation on North Queensland environments: management and remediation © James Cook University ISBN 978-1-921359-12-5 This report should be cited as: Pohlman, C. and Goosem, M. (2007) The effects of Severe Tropical Cyclone Larry on rainforest vegetation and understorey microclimate adjacent to powerlines, highways and streams in the Wet Tropics World Heritage Area. Report to the Marine and Tropical Sciences Research Facility. Reef and Rainforest Research Centre Limited, Cairns (47pp.). Made available online by the Reef and Rainforest Research Centre on behalf of the Australian Government’s Marine and Tropical Sciences Research Facility. The Australian Government’s Marine and Tropical Sciences Research Facility (MTSRF) supports world-class, public good research. The MTSRF is a major initiative of the Australian Government, designed to ensure that Australia’s environmental challenges are addressed in an innovative, collaborative and sustainable way. The MTSRF investment is managed by the Department of the Environment, Water, Heritage and the Arts (DEWHA), and is supplemented by substantial cash and in-kind investments from research providers and interested third parties. The Reef and Rainforest Research Centre Limited (RRRC) is contracted by DEWHA to provide program management and communications services for the MTSRF. This publication is copyright. The Copyright Act 1968 permits fair dealing for study, research, information or educational purposes subject to inclusion of a sufficient acknowledgement of the source. -

Juglandaceae (Walnuts)
A start for archaeological Nutters: some edible nuts for archaeologists. By Dorian Q Fuller 24.10.2007 Institute of Archaeology, University College London A “nut” is an edible hard seed, which occurs as a single seed contained in a tough or fibrous pericarp or endocarp. But there are numerous kinds of “nuts” to do not behave according to this anatomical definition (see “nut-alikes” below). Only some major categories of nuts will be treated here, by taxonomic family, selected due to there ethnographic importance or archaeological visibility. Species lists below are not comprehensive but representative of the continental distribution of useful taxa. Nuts are seasonally abundant (autumn/post-monsoon) and readily storable. Some good starting points: E. A. Menninger (1977) Edible Nuts of the World. Horticultural Books, Stuart, Fl.; F. Reosengarten, Jr. (1984) The Book of Edible Nuts. Walker New York) Trapaceae (water chestnuts) Note on terminological confusion with “Chinese waterchestnuts” which are actually sedge rhizome tubers (Eleocharis dulcis) Trapa natans European water chestnut Trapa bispinosa East Asia, Neolithic China (Hemudu) Trapa bicornis Southeast Asia and South Asia Trapa japonica Japan, jomon sites Anacardiaceae Includes Piastchios, also mangos (South & Southeast Asia), cashews (South America), and numerous poisonous tropical nuts. Pistacia vera true pistachio of commerce Pistacia atlantica Euphorbiaceae This family includes castor oil plant (Ricinus communis), rubber (Hevea), cassava (Manihot esculenta), the emblic myrobalan fruit (of India & SE Asia), Phyllanthus emblica, and at least important nut groups: Aleurites spp. Candlenuts, food and candlenut oil (SE Asia, Pacific) Archaeological record: Late Pleistocene Timor, Early Holocene reports from New Guinea, New Ireland, Bismarcks; Spirit Cave, Thailand (Early Holocene) (Yen 1979; Latinis 2000) Rincinodendron rautanenii the mongongo nut, a Dobe !Kung staple (S. -

Cryptocarya Nitens (Lauraceae), a New Species Record for Singapore
Gardens’ Bulletin Singapore 67(2): 253–259. 2015 253 doi: 10.3850/S2382581215000204 Cryptocarya nitens (Lauraceae), a new species record for Singapore R.P.J. de Kok 275 Cricklewood Lane, London, NW2 2JJ, U.K. [email protected] ABSTRACT. Cryptocarya nitens (Blume) Koord. & Valeton is newly recorded for Singapore. It was discovered during surveys in Bukit Timah Nature Reserve and the Nee Soon Swamp Forest. A description is given, together with a key based mostly on vegetative characters for all Cryptocarya species occurring in Singapore. A short overview of the Lauraceae of Singapore shows that, in total, 57 species belonging to 14 genera have been recorded, of which 47 species in 13 genera are native. Cryptocarya nitens is lectotypified in addition to two of its synonyms. Keywords. Cryptocarya, Endiandra Introduction Species of the Lauraceae (Laurel Family) are of major ecological and economic importance in Southeast Asia as they comprise a major part of almost any forest in the region. The family is very complex, posing many challenging taxonomic and systematic questions, and for various reasons the identification of species is problematic. Some systematic work has been done, for instance Kostermans (1952, 1957a, 1957b, 1964) wrote a series of papers on various genera or parts of genera during his career, and he also dealt with some aspects of the higher taxonomy. The most recent overview of the family as a whole was by Rohwer (1993). In Eastern Asia and Australasia, only for Peninsular Malaysia (Kochummen, 1989), China (Li Xiwen et al., 2008) and Australia (Le Cussan & Hyland, 2007) are recent treatments available, dealing with all species occurring in their territories. -

I Is the Sunda-Sahul Floristic Exchange Ongoing?
Is the Sunda-Sahul floristic exchange ongoing? A study of distributions, functional traits, climate and landscape genomics to investigate the invasion in Australian rainforests By Jia-Yee Samantha Yap Bachelor of Biotechnology Hons. A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2018 Queensland Alliance for Agriculture and Food Innovation i Abstract Australian rainforests are of mixed biogeographical histories, resulting from the collision between Sahul (Australia) and Sunda shelves that led to extensive immigration of rainforest lineages with Sunda ancestry to Australia. Although comprehensive fossil records and molecular phylogenies distinguish between the Sunda and Sahul floristic elements, species distributions, functional traits or landscape dynamics have not been used to distinguish between the two elements in the Australian rainforest flora. The overall aim of this study was to investigate both Sunda and Sahul components in the Australian rainforest flora by (1) exploring their continental-wide distributional patterns and observing how functional characteristics and environmental preferences determine these patterns, (2) investigating continental-wide genomic diversities and distances of multiple species and measuring local species accumulation rates across multiple sites to observe whether past biotic exchange left detectable and consistent patterns in the rainforest flora, (3) coupling genomic data and species distribution models of lineages of known Sunda and Sahul ancestry to examine landscape-level dynamics and habitat preferences to relate to the impact of historical processes. First, the continental distributions of rainforest woody representatives that could be ascribed to Sahul (795 species) and Sunda origins (604 species) and their dispersal and persistence characteristics and key functional characteristics (leaf size, fruit size, wood density and maximum height at maturity) of were compared. -

Budawangia* an E-Newsletter for All Those Interested in the Native Plants of the Nsw South Coast
BUDAWANGIA* AN E-NEWSLETTER FOR ALL THOSE INTERESTED IN THE NATIVE PLANTS OF THE NSW SOUTH COAST Contact: Dr Kevin Mills – [email protected] No. 32 - November 2014 Aims: To connect those interested in the native flora of the NSW South Coast, to share up to date information on the flora of the region and to broaden the appreciation of the region’s native plants. Editorial There are so many rainforest trees that many people find it difficult to accurately identify individual species based on leaves alone. This is true of the trees in the laurel family (Lauraceae). This edition contains a piece on identifying the local species based on leaf characteristics. Also included, is a note on a new record of a small rainforest tree found at Minnamurra Rainforest. This edition includes a new mystery weed, a piece on the Giant Pepper Vine of the local rainforests and wetland plant No. 8. Once again, the Illawarra Flame Trees Brachychiton acerifolius are flowering well in the region. Blotches of red appeared along the escarpment and in the rainforest remnants around Kiama early in the month. The common name flame tree is well deserved, as the bright red colour of thousands of flowers really does look like a tree in flame; this is heightened by the fact that the trees are usually leafless at this time. The genus Brachychiton is derived from brachys for short and chiton, a coat of mail, in allusion to the bristly coasting around the seeds in this genus. The species name acerifolius is in reference to the leaves that at times look like those in the deciduous maple tree genus Acer. -

On the Flora of Australia
L'IBRARY'OF THE GRAY HERBARIUM HARVARD UNIVERSITY. BOUGHT. THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEING AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. r^/f'ORElGN&ENGLISH' <^ . 1859. i^\BOOKSELLERS^.- PR 2G 1.912 Gray Herbarium Harvard University ON THE FLORA OF AUSTRALIA ITS ORIGIN, AFFINITIES, AND DISTRIBUTION. I I / ON THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEIKG AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. Reprinted from the JJotany of the Antarctic Expedition, Part III., Flora of Tasmania, Vol. I. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. 1859. PRINTED BY JOHN EDWARD TAYLOR, LITTLE QUEEN STREET, LINCOLN'S INN FIELDS. CONTENTS OF THE INTRODUCTORY ESSAY. § i. Preliminary Remarks. PAGE Sources of Information, published and unpublished, materials, collections, etc i Object of arranging them to discuss the Origin, Peculiarities, and Distribution of the Vegetation of Australia, and to regard them in relation to the views of Darwin and others, on the Creation of Species .... iii^ § 2. On the General Phenomena of Variation in the Vegetable Kingdom. All plants more or less variable ; rate, extent, and nature of variability ; differences of amount and degree in different natural groups of plants v Parallelism of features of variability in different groups of individuals (varieties, species, genera, etc.), and in wild and cultivated plants vii Variation a centrifugal force ; the tendency in the progeny of varieties being to depart further from their original types, not to revert to them viii Effects of cross-impregnation and hybridization ultimately favourable to permanence of specific character x Darwin's Theory of Natural Selection ; — its effects on variable organisms under varying conditions is to give a temporary stability to races, species, genera, etc xi § 3.