Haustorium Initiation and Early Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wild Plants of Round Valley Regional Preserve Common Name Version
Wild Plants of Round Valley Regional Preserve Common Name Version A Photographic Guide Sorted by Form, Color and Family with Habitat Descriptions and Identification Notes Photographs and text by Wilde Legard District Botanist, East Bay Regional Park District New Revised and Expanded Edition - Includes the latest scientific names, habitat descriptions and identification notes Decimal Inches .1 .2 .3 .4 .5 .6 .7 .8 .9 1 .5 2 .5 3 .5 4 .5 5 .5 6 .5 7 .5 8 .5 9 1/8 1/4 1/2 3/4 1 1/2 2 1/2 3 1/2 4 1/2 5 1/2 6 1/2 7 1/2 8 1/2 9 English Inches Notes: A Photographic Guide to the Wild Plants of Round Valley Regional Preserve More than 2,000 species of native and naturalized plants grow wild in the San Francisco Bay Area. Most are very difficult to identify without the help of good illustrations. This is designed to be a simple, color photo guide to help you identify some of these plants. This guide is published electronically in Adobe Acrobat® format so that it can easily be updated as additional photographs become available. You have permission to freely download, distribute and print this guide for individual use. Photographs are © 2014 Wilde Legard, all rights reserved. In this guide, the included plants are sorted first by form (Ferns & Fern-like, Grasses & Grass-like, Herbaceous, Woody), then by most common flower color, and finally by similar looking flowers (grouped by genus within each family). Each photograph has the following information, separated by '-': COMMON NAME According to The Jepson Manual: Vascular Plants of California, Second Edition (JM2) and other references (not standardized). -

Appendix C. Plant Species Observed at the Yolo Grasslands Regional Park (2009-2010)
Appendix C. Plant Species Observed at the Yolo Grasslands Regional Park (2009-2010) Plant Species Observed at the Yolo Grassland Regional Park (2009-2010) Wetland Growth Indicator Scientific Name Common Name Habitat Occurrence Habit Status Family Achyrachaena mollis Blow wives AG, VP, VS AH FAC* Asteraceae Aegilops cylinricia* Jointed goatgrass AG AG NL Poaceae Aegilops triuncialis* Barbed goat grass AG AG NL Poaceae Aesculus californica California buckeye D T NL Hippocastanaceae Aira caryophyllea * [Aspris c.] Silver hairgrass AG AG NL Poaceae Alchemilla arvensis Lady's mantle AG AH NL Rosaceae Alopecurus saccatus Pacific foxtail VP, SW AG OBL Poaceae Amaranthus albus * Pigweed amaranth AG, D AH FACU Amaranthaceae Amsinckia menziesii var. intermedia [A. i.] Rancher's fire AG AH NL Boraginaceae Amsinckia menziesii var. menziesii Common fiddleneck AG AH NL Boraginaceae Amsinckia sp. Fiddleneck AG, D AH NL Boraginaceae Anagallis arvensis * Scarlet pimpernel SW, D, SS AH FAC Primulaceae Anthemis cotula * Mayweed AG AH FACU Asteraceae Anthoxanthum odoratum ssp. odoratum * Sweet vernal grass AG PG FACU Poaceae Aphanes occidentalis [Alchemilla occidentalis] Dew-cup AG, F AH NL Rosaceae Asclepias fascicularis Narrow-leaved milkweed AG PH FAC Ascepiadaceae Atriplex sp. Saltbush VP, SW AH ? Chenopodiaceae Avena barbata * Slender wild oat AG AG NL Poaceae Avena fatua * [A. f. var. glabrata, A. f. var. vilis] Wild oat AG AG NL Poaceae Brassica nigra * Black mustard AG, D AH NL Brassicaceae Brassica rapa field mustard AG, D AH NL Brassicaceae Briza minor * Little quakinggrass AG, SW, SS, VP AG FACW Poaceae Brodiaea californica California brodiaea AG PH NL Amaryllidaceae Brodiaea coronaria ssp. coronaria [B. -
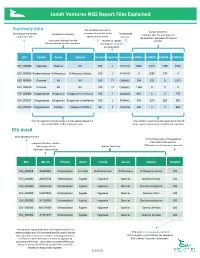
NGS Report Explained
Jonah Ventures NGS Report Files Explained Summary data The % of base pairs in the Sample identifiers Exact Sequence Variant sequence that match to the The detected Exact Sequence Variant Consensus Taxonomy The detected Numbers after the decimal point unique identifier species in the library. sequence. represent lab replicates of the same Taxonomic ranking from the Number of species sample. library matched to each sequence. matching the sequence at a given level. ESV Family Genus Species %match # Species Sequence S10001.1 S10002.1 S10003.1 S10003.2 ESV_000080 Fagaceae Quercus NA 100 7 AAAAAG… 3066 1340 5780 3462 ESV_000818 Orobanchaceae Orthocarpus Orthocarpus luteus 100 1 AAAAAG… 0 2582 249 0 ESV_000006 Poaceae NA NA 100 77 GAAAAG… 584 101 0 1039 ESV_000050 Poaceae NA NA 100 12 GAAAAG… 1328 0 0 0 ESV_000308 Polygonaceae Polygonum Polygonum humifusum 100 2 AAAAAG… 902 0 0 278 ESV_000027 Polygonaceae Eriogonum Eriogonum umbellatum 100 1 ATAAAG… 341 124 282 381 ESV_000520 Polygonaceae Fallopia Fallopia multiflora 99 1 AAAAAG… 126 0 0 994 “NA” at a taxonomic ranks means multiple species present in The number in each cell is the absolute number of the sample differ in that taxonomic rank times a given sequence was read by the sequencer. ESV detail Exact Sequence Variant The % of base pairs in the sequence that match to the species. Index identification number. that match to the species. Each sample has an Species Taxonomy 100% indicates all base pairs matched individual index number. ESV GB_ID Phylum Order Family Genus Species %match ESV_000818 -

103 Pedicularis, Orthocarpus (Scrophulariaceae), and Plantago
VOLUME 28, NUMBER 2 103 GEOGRAPHICAL DISTRIBUTION OF HOSTPLANT CHOICE IN EUPHYDRYAS EDITHA (NYMPHALIDAE) RAYMOND R. WHITE AND MICHAEL C. SINGER Department of Biological Sciences, Stanford University, Stanford, California 94305 An investigation of populations of Euphydryas editha Boisduval re veals a disjunct distribution of food plant choice (Fig. 1). Euphydryas editha may oviposit on plants of at least five genera: Collinsia, Castilleja, Pedicularis, Orthocarpus (Scrophulariaceae), and Plantago (Plantagina ceae). With rare exceptions, only a single plant species is selected in each population, even though plants that are selected elsewhere may be abundant. This parallels observations of Downey & Fuller (1961) on Plebejus icarioides Boisduva!. We have visited as many Euphydryas populations as possible, identifying primary hostplants of 50 by observing oviposition or by locating eggs or webs of prediapause larvae. Post diapause larvae may move onto secondary foodplants and may even prefer these to primary hosts (Table 1). Oviposition preference in the laboratory is not necessarily the same as that in the field, and cannot always be used as positive evidence for placing a population in a par ticular foodplant category. Our present knowledge of the distribution of hostplant choice (mostly in California) is summarised in Fig. 1. Though it is difficult to separate cause and effect, there are strong correlations between plant species chosen and a) timing of flight season, and b) type of community in habited. Early-flying, coastal populations are Plantago-feeding, with some oviposition on Orthocarpus (EW, WS) and fewer on Collinsia ( CS ). Low altitude, late-flying populations in the chaparral belt of the Inner Coast Ranges are all on serpentine soils and utilise Pedicularis densiflora Benth. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Anthony Chabot Plants
Anthony Chabot Plants A photographic guide to wild plants of Anthony Chabot Regional Park Sorted by Scientific Name Photographs by Wilde Legard Botanist, East Bay Regional Park District Revision: February 23, 2007 More than 2,000 species of native and naturalized plants grow wild in the San Francisco Bay Area. Most are very difficult to identify without the help of good illustrations. This is designed to be a simple, color photo guide to help you identify some of these plants. The selection of plants displayed in this guide is by no means complete. The intent is to expand the quality and quantity of photos over time. The revision date is shown on the cover and on the header of each photo page. A comprehensive plant list for this area (including the many species not found in this publication) can be downloaded at the East Bay Regional Park District’s wild plant download page at: http://www.ebparks.org. This guide is published electronically in Adobe Acrobat® format to accommodate these planned updates. You have permission to freely download, distribute, and print this pdf for individual use. You are not allowed to sell the electronic or printed versions. In this version of the guide, the included plants are sorted alphabetically by scientific name. Under each photograph are four lines of information, based on upon the current standard wild plant reference for California: The Jepson Manual: Higher Plants of California, 1993. Scientific Name Scientific names revised since 1993 are NOT included in this edition. Common Name These non-standard names are based on Jepson and other local references. -

Western Bumblebee Surveys, Rogue River-Siskiyou National Forest
2016 Western Bumble Bee Surveys: Rogue River-Siskiyou National Forest Bombus occidentalis found on Mt. Ashland (photo credit: Bonnie Allison) Sheila M. Colyer 5- December 2016 2016 Western Bumble Bee Surveys: Rogue River-Siskiyou National Forest Species Status: Bombus occidentalis (Western Bumble bee) G2G3, S1S2 R6 Regional Forester’s Sensitive Species (USFS) Oregon State Director’s Sensitive Species (BLM) State of Oregon – NA ORBIC List 2 District Contacts: Bonnie Allison Zoned Wildlife Biologist, Siskiyou Mountains Ranger District and Wild Rivers Ranger District Rachael Vaughn Wildlife Biologist, Powers Ranger District and Gold Beach Ranger District Sheila Colyer Wildlife Biologist, High Cascades Ranger District Abstract The Rogue River-Siskiyou National Forest conducted surveys for Western bumble bee (Bombus occidentalis) during the 2016 field season at 15 sites across the Forest. Surveys were primarily concentrated on historic locations, in meadow habitat and open roadside. One location of Bombus occidentalis was observed on Mt. Ashland (Siskiyou Mountains Ranger District). In addition, 14 total Bombus species were observed across all sites. Additional surveys for the Forest are recommended primarily focused on more historic locations. 2016 Western Bumble Bee Surveys: Rogue River-Siskiyou National Forest 1 Introduction Bombus occidentalis (hereafter B. occidentalis) was historically widely distributed across the west coast of North America from Alaska to central California, east through Alberta and western South Dakota, and south to Arizona and New Mexico (Williams et al. 2014). A generalist forager and native pollinator, this species and many other Bombus species play an integral role in the health of natural ecosystems and production of agricultural crops (Cameron 2011). -

COSEWIC Assessment and Status Report on the Victoria Owl-Clover In
COSEWIC Assessment and Status Report on the Victoria’s Owl-clover Castilleja victoriae in Canada ENDANGERED 2010 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2010. COSEWIC assessment and status report on the Victoria’s Owl-clover Castilleja victoriae in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 20 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge Matt Fairbarns for writing the status report on the Victoria’s Owl- clover, Castilleja victoriae, in Canada. COSEWIC also gratefully acknowledges the financial support of the BC Conservation Data Centre and Parks Canada for the preparation of this report. The COSEWIC report review was overseen by Erich Haber, Co-chair, COSEWIC Vascular Plants Species Specialist Subcommittee, with input from members of COSEWIC. That review may have resulted in changes and additions to the initial version of the report. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le castilléjie de Victoria (Castilleja victoriae) au Canada. Cover illustration/photo: Victoria’s Owl-clover — Photo by Matt Fairbarns. ©Her Majesty the Queen in Right of Canada, 2010. Catalogue CW69-14/610-2010E-PDF ISBN 978-1-100-16058-0 Recycled paper COSEWIC Assessment Summary Assessment Summary – April 2010 Common name Victoria’s Owl-clover Scientific name Castilleja victoriae Status Endangered Reason for designation This small annual herb is confined to a very small area of British Columbia and one site in adjacent Washington State. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. -

An Illustrated Key to the Alberta Figworts & Allies
AN ILLUSTRATED KEY TO THE ALBERTA FIGWORTS & ALLIES OROBANCHACEAE PHRYMACEAE PLANTAGINACEAE SCROPHULARIACEAE Compiled and writen by Lorna Allen & Linda Kershaw April 2019 © Linda J. Kershaw & Lorna Allen Key to Figwort and Allies Families In the past few years, the families Orobanchaceae, Plantaginaceae and Scrophulariaceae have under- gone some major revision and reorganization. Most of the species in the Scrophulariaceae in the Flora of Alberta (1983) are now in the Orobanchaceae and Plantaginaceae. For this reason, we’ve grouped the Orobanchaceae, Plantaginaceae, Phrymaceae and Scrophulariaceae together in this fle. In addition, species previously placed in the Callitrichaceae and Hippuridaceae families are now included in the Plantaginaceae family. 01a Plants aquatic, with many or all leaves submersed and limp when taken from the 1a water; leaves paired or in rings (whorled) on the stem, all or mostly linear (foating leaves sometimes spatula- to egg-shaped); fowers tiny (1-2 mm), single or clustered in leaf axils; petals and sepals absent or sepals fused in a cylinder around the ovary; stamens 0-1 . Plantaginaceae (in part) . - Callitriche, Hippuris 01b Plants emergent wetland species (with upper stems and leaves held above water) or upland species with self-supporting stems and leaves; leaves not as above; fowers larger, single or in clusters; petals and sepals present; stamens 2-4 (Hippuris sometimes emergent, but leaves/ fowers distinctive) . .02 2a 02a Plants without green leaves . Orobanchaceae (in part) . - Aphyllon [Orobanche], Boschniakia 02b Plants with green leaves . 03 03a Leaves all basal (sometimes small, unstalked stem leaves present), undivided (simple), with edges ± smooth or blunt-toothed; fowers small (2-5 mm wide), corollas radially symmetrical, sometimes absent. -

Open Yetingdissertationori.Pdf
The Pennsylvania State University The Graduate School Intercollege Graduate Program in Genetics HORIZONTAL GENE TRANSFER STUDIES IN PARASITIC PLANTS OF THE OROBANCHACEAE A Dissertation in Genetics by Yeting Zhang © 2013 Yeting Zhang Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2013 The dissertation of Yeting Zhang was reviewed and approved* by the following: Stephen W. Schaeffer Professor of Biology Chair of Committee Claude W. dePamphilis Professor of Biology Dissertation Advisor Tomas A. Carlo Assistant Professor of Biology John E. Carlson Professor of Molecular Genetics, School of Forest Resources Director of The Schatz Center for Tree Molecular Genetics Naomi Altman Professor of Statistics Robert F. Paulson Professor of Veterinary and Biomedical Sciences Chair, Intercollege Graduate Degree Program in Genetics *Signatures are on file in the Graduate School ii ABSTRACT Parasitic plants, represented by several thousand species of angiosperms, use modified structures known as haustoria to tap into photosynthetic host plants in order to extract nutrients and water. As a result of these direct plant-to-plant connections with their host plants, parasitic plants have unique opportunities for horizontal gene transfer (HGT), the nonsexual transmission of genetic material across species boundaries. There is increasing evidence that parasitic plants have served as the recipients and donors of HGT, but the long-term impacts of eukaryotic HGT in parasitic plants are largely unknown. Three parasitic plant genera from Orobanchaceae (Triphysaria versicolor, Striga hermonthica, and Orobanche aegyptiaca (syn. Phelipanche aegyptiaca)) were chosen for a massive transcriptome-sequencing project known as the Parasitic Plant Genome Project (PPGP). These species were chosen for two reasons. -

A Synoptical Classification of the Lamiales
Lamiales – Synoptical classification vers. 2.0 (in prog.) Updated: 13 December, 2005 A Synoptical Classification of the Lamiales Version 2.0 (in progress) Compiled by Richard Olmstead With the help of: D. Albach, B. Bremer, P. Cantino, C. dePamphilis, P. Garnock-Jones, R. Harley, L. McDade, E. Norman, B. Oxelman, J. Reveal, R. Scotland, J. Smith, E. Wallander, A. Weber, A. Wolfe, N. Young, M. Zjhra, and others [estimated # species in Lamiales = 22,000] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near-term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. Lamiales – Synoptical classification vers. 2.0 (in prog.) Updated: 13 December, 2005 Acanthaceae (~201/3510) Durande, Notions Elém. Bot.: 265. 1782, nom. cons. – Synopsis compiled by R. Scotland & K. Vollesen (Kew Bull. 55: 513-589. 2000); probably should include Avicenniaceae. Nelsonioideae (7/ ) Lindl. ex Pfeiff., Nomencl.