COSEWIC Assessment and Status Report on the Victoria Owl-Clover In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Castilleja Coccinea and C. Indivisa (Orobanchaceae)
Nesom, G.L. and J.M. Egger. 2014. Castilleja coccinea and C. indivisa (Orobanchaceae). Phytoneuron 2014-14: 1–7. Published 6 January 2014. ISSN 2153 733X CASTILLEJA COCCINEA AND C. INDIVISA (OROBANCHACEAE) GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 www.guynesom.com J. M ARK EGGER Herbarium, Burke Museum of Natural History and Culture University of Washington Seattle, Washington 98195-5325 [email protected] ABSTRACT Castilleja coccinea and C. indivisa are contrasted in morphology and their ranges mapped in detail in the southern USA, where they are natively sympatric in small areas of Oklahoma, Arkansas, and Louisiana. Castilleja indivisa has recently been introduced and naturalized in the floras of Alabama and Florida. Castilleja ludoviciana , known only by the type collection from southwestern Louisiana, differs from C. coccinea in subentire leaves and relatively small flowers and is perhaps a population introgressed by C. indivisa . Castilleja coccinea and C. indivisa are allopatric except in small areas of Oklahoma, Arkansas, and Lousiana, but assessments of their native distributions are not consistent among various accounts (e.g. Thomas & Allen 1997; Turner et al. 2003; OVPD 2012; USDA, NRCS 2013). Morphological contrasts between the two species, via keys in floristic treatments (e.g., Smith 1994; Wunderlin & Hansen 2003; Nelson 2009; Weakley 2012), have essentially repeated the differences outlined by Pennell (1935). The current study presents an evaluation and summary of the taxonomy of these two species. We have examined specimens at CAS, TEX-LL, SMU-BRIT-VDB, MO, NLU, NO, USF, WS, and WTU and viewed digital images available through Florida herbaria and databases. -

Molecular Investigation of the Origin of Castilleja Crista-Galli by Sarah
Molecular investigation of the origin of Castilleja crista-galli by Sarah Youngberg Mathews A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences Montana State University © Copyright by Sarah Youngberg Mathews (1990) Abstract: An hypothesis of hybrid origin of Castilleja crista-galli (Scrophulariaceae) was studied. Hybridization and polyploidy are widespread in Castilleja and are often invoked as a cause of difficulty in defining species and as a speciation model. The putative allopolyploid origin of Castilleja crista-gralli from Castilleja miniata and Castilleja linariifolia was investigated using molecular, morphological and cytological techniques. Restriction site analysis of chloroplast DNA revealed high homogeneity among the chloroplast genomes of species of Castilleja and two Orthocarpus. No species of Castilleia represented by more than one population in the analysis was characterized by a distinctive choroplast genome. Genetic distances estimated from restriction site mutations between any two species or between genera are comparable to distances reported from other plant groups, but both intraspecific and intrapopulational distances are high relative to other groups. Restriction site analysis of nuclear ribosomal DNA revealed variable repeat types both within and among individuals. Qualitative species groupings based on restriction site mutations in the ribosomal DNA repeat units do not place Castilleja crista-galli with either putative parent in a consistent manner. A cladistic analysis of 11 taxa using 10 morphological characters places Castilleja crista-galli in an unresolved polytomy with both putative parents and Castilleja hispida. Cytological analyses indicate that Castilleja crista-gralli is not of simple allopolyploid origin. Both diploid and tetraploid chromosome counts are reported for this species, previously known only as an octoploid. -

The Effects of Hemiparasitism by Castilleja Species on Community Structure in Alpine Ecosystems
Pursuit - The Journal of Undergraduate Research at The University of Tennessee Volume 3 Issue 2 Spring 2012 Article 8 March 2012 The Effects of Hemiparasitism by Castilleja Species on Community Structure in Alpine Ecosystems Johannah Reed University of Tennessee, [email protected] Follow this and additional works at: https://trace.tennessee.edu/pursuit Recommended Citation Reed, Johannah (2012) "The Effects of Hemiparasitism by Castilleja Species on Community Structure in Alpine Ecosystems," Pursuit - The Journal of Undergraduate Research at The University of Tennessee: Vol. 3 : Iss. 2 , Article 8. Available at: https://trace.tennessee.edu/pursuit/vol3/iss2/8 This Article is brought to you for free and open access by Volunteer, Open Access, Library Journals (VOL Journals), published in partnership with The University of Tennessee (UT) University Libraries. This article has been accepted for inclusion in Pursuit - The Journal of Undergraduate Research at The University of Tennessee by an authorized editor. For more information, please visit https://trace.tennessee.edu/pursuit. Pursuit: The Journal of Undergraduate Research at the University of Tennessee Copyright © The University of Tennessee The Effects of Hemiparasitism by Castilleja Species on Community Structure in Alpine Ecosystems JOHANNAH REED Advisor: Nate Sanders Environmental Studies, University of Tennessee, Knoxville There is a long history in ecology of examining how interactions such as competition, predation, and mutualism influence the structure and dynamics of natural communities. However, few studies to date have experimentally as- sessed the role of hemiparasitic plants as a structuring force. Hemiparasitic plants have the potential to shape plant communities because of their ability to photosynthesize and parasitize and because of their abundance in a variety of natural and managed ecosystems. -
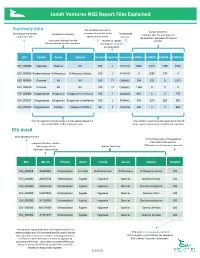
NGS Report Explained
Jonah Ventures NGS Report Files Explained Summary data The % of base pairs in the Sample identifiers Exact Sequence Variant sequence that match to the The detected Exact Sequence Variant Consensus Taxonomy The detected Numbers after the decimal point unique identifier species in the library. sequence. represent lab replicates of the same Taxonomic ranking from the Number of species sample. library matched to each sequence. matching the sequence at a given level. ESV Family Genus Species %match # Species Sequence S10001.1 S10002.1 S10003.1 S10003.2 ESV_000080 Fagaceae Quercus NA 100 7 AAAAAG… 3066 1340 5780 3462 ESV_000818 Orobanchaceae Orthocarpus Orthocarpus luteus 100 1 AAAAAG… 0 2582 249 0 ESV_000006 Poaceae NA NA 100 77 GAAAAG… 584 101 0 1039 ESV_000050 Poaceae NA NA 100 12 GAAAAG… 1328 0 0 0 ESV_000308 Polygonaceae Polygonum Polygonum humifusum 100 2 AAAAAG… 902 0 0 278 ESV_000027 Polygonaceae Eriogonum Eriogonum umbellatum 100 1 ATAAAG… 341 124 282 381 ESV_000520 Polygonaceae Fallopia Fallopia multiflora 99 1 AAAAAG… 126 0 0 994 “NA” at a taxonomic ranks means multiple species present in The number in each cell is the absolute number of the sample differ in that taxonomic rank times a given sequence was read by the sequencer. ESV detail Exact Sequence Variant The % of base pairs in the sequence that match to the species. Index identification number. that match to the species. Each sample has an Species Taxonomy 100% indicates all base pairs matched individual index number. ESV GB_ID Phylum Order Family Genus Species %match ESV_000818 -

Recovery Strategy for Multi-Species at Risk in Vernal Pools and Other Ephemeral Wet Areas Associated with Garry Oak Ecosystems in Canada
Species at Risk Act Recovery Strategy Series Recovery Strategy for Multi-Species at Risk in Vernal Pools and other Ephemeral Wet Areas Associated with Garry Oak Ecosystems in Canada Bog birds-foot trefoil Tall woolly-heads (Pacific population) Water plantain buttercup Kellogg’s rush Rosy owl clover Dwarf sandwort July 2006 About the Species at Risk Act Recovery Strategy Series What is the Species at Risk Act (SARA)? SARA is the Act developed by the federal government as a key contribution to the common national effort to protect and conserve species at risk in Canada. SARA came into force in 2003 and one of its purposes is “to provide for the recovery of wildlife species that are extirpated, endangered or threatened as a result of human activity.” What is recovery? In the context of species at risk conservation, recovery is the process by which the decline of an endangered, threatened or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of the species’ persistence in the wild. A species will be considered recovered when its long-term persistence in the wild has been secured. What is a recovery strategy? A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets goals and objectives and identifies the main areas of activities to be undertaken. Detailed planning is done at the action plan stage. Recovery strategy development is a commitment of all provinces and territories and of three federal agencies — Environment Canada, Parks Canada Agency and Fisheries and Oceans Canada — under the Accord for the Protection of Species at Risk. -

103 Pedicularis, Orthocarpus (Scrophulariaceae), and Plantago
VOLUME 28, NUMBER 2 103 GEOGRAPHICAL DISTRIBUTION OF HOSTPLANT CHOICE IN EUPHYDRYAS EDITHA (NYMPHALIDAE) RAYMOND R. WHITE AND MICHAEL C. SINGER Department of Biological Sciences, Stanford University, Stanford, California 94305 An investigation of populations of Euphydryas editha Boisduval re veals a disjunct distribution of food plant choice (Fig. 1). Euphydryas editha may oviposit on plants of at least five genera: Collinsia, Castilleja, Pedicularis, Orthocarpus (Scrophulariaceae), and Plantago (Plantagina ceae). With rare exceptions, only a single plant species is selected in each population, even though plants that are selected elsewhere may be abundant. This parallels observations of Downey & Fuller (1961) on Plebejus icarioides Boisduva!. We have visited as many Euphydryas populations as possible, identifying primary hostplants of 50 by observing oviposition or by locating eggs or webs of prediapause larvae. Post diapause larvae may move onto secondary foodplants and may even prefer these to primary hosts (Table 1). Oviposition preference in the laboratory is not necessarily the same as that in the field, and cannot always be used as positive evidence for placing a population in a par ticular foodplant category. Our present knowledge of the distribution of hostplant choice (mostly in California) is summarised in Fig. 1. Though it is difficult to separate cause and effect, there are strong correlations between plant species chosen and a) timing of flight season, and b) type of community in habited. Early-flying, coastal populations are Plantago-feeding, with some oviposition on Orthocarpus (EW, WS) and fewer on Collinsia ( CS ). Low altitude, late-flying populations in the chaparral belt of the Inner Coast Ranges are all on serpentine soils and utilise Pedicularis densiflora Benth. -

TAXONOMY Plant Family Species Scientific Name
CAEL7 1 Plant Propagation Protocol for Castilleja elmeri ESRM 412 – Native Plant Production Protocol URL: https://courses.washington.edu/esrm412/protocols/[USDASpeciesCode.pdf] Castilleja elmeri, Wenatchee Indian paintbrush (Source: Walter Siegmund1) TAXONOMY Plant Family Scientific Name Scrophulariaceae Common Name Figwort family Species Scientific Name Scientific Name Castilleja elmeri (Fernald) Varieties N/A Sub-species N/A Cultivar N/A Common Synonym(s) Castilleja angustifolia (G. Don) var. whitedii Piper2 Common Name(s) Wenatchee Indian paintbrush3, Elmer’s paintbrush2 Species Code (as per USDA CAEL7 Plants database) CAEL7 2 GENERAL INFORMATION Geographical range North America Distribution Washington State Distribution Source: USDA Plants Database3 Wenatchee Mountains and the east slope of the Cascades, Kittitas County, Washington; north into British Columbia2 Ecological distribution Moist, open slopes at mid-elevations in the mountains2 Climate and elevation range Mid-elevation4 Local habitat and abundance Found near sedges and fescues, commonly using them as hosts for hemi-parasitic roots5 Plant strategy type / Hemi-parasitic5 (capable of manufacturing their own food and successional stage obtaining water/ nutrients from soil, but also form specialized roots—haustoria roots—that attach to a host plant to take up additional water); herb3 CAEL7 3 Plant characteristics Perennial species; blooms June-August2; some reports that Castilleja seed is difficult to germinate and that chemical exudate from the roots of host species (Castilleja are parasitic) are needed to induce germination, however this pattern is not always observed6; pollinated by insects and hummingbirds5 PROPAGATION DETAILS Ecotype N/A Propagation Goal Plants Propagation Method Seed Product Type Container (plug) Stock Type N/A Time to Grow 16 weeks5 Target Specifications Hardened 16-wk-old plants Propagule Collection Seeds can be collected in midsummer for early spring flowering Instructions species and in late summer for mid-elevation species. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Vascular Plant Species with Documented Or Recorded Occurrence in Placer County
A PPENDIX II Vascular Plant Species with Documented or Reported Occurrence in Placer County APPENDIX II. Vascular Plant Species with Documented or Reported Occurrence in Placer County Family Scientific Name Common Name FERN AND FERN ALLIES Azollaceae Mosquito fern family Azolla filiculoides Pacific mosquito fern Dennstaedtiaceae Bracken family Pteridium aquilinum var.pubescens Bracken fern Dryopteridaceae Wood fern family Athyrium alpestre var. americanum Alpine lady fern Athyrium filix-femina var. cyclosorum Lady fern Cystopteris fragilis Fragile fern Polystichum imbricans ssp. curtum Cliff sword fern Polystichum imbricans ssp. imbricans Imbricate sword fern Polystichum kruckebergii Kruckeberg’s hollyfern Polystichum lonchitis Northern hollyfern Polystichum munitum Sword fern Equisetaceae Horsetail family Equisetum arvense Common horsetail Equisetum hyemale ssp. affine Scouring rush Equisetum laevigatum Smooth horsetail Isoetaceae Quillwort family Isoetes bolanderi Bolander’s quillwort Isoetes howellii Howell’s quillwort Isoetes orcuttii Orcutt’s quillwort Lycopodiaceae Club-moss family Lycopodiella inundata Bog club-moss Marsileaceae Marsilea family Marsilea vestita ssp. vestita Water clover Pilularia americana American pillwort Ophioglossaceae Adder’s-tongue family Botrychium multifidum Leathery grapefern Polypodiaceae Polypody family Polypodium hesperium Western polypody Pteridaceae Brake family Adiantum aleuticum Five-finger maidenhair Adiantum jordanii Common maidenhair fern Aspidotis densa Indian’s dream Cheilanthes cooperae Cooper’s -

A Comparative Study of the Floras of China and Canada
Núm. 24, pp. 25-51, ISSN 1405-2768; México, 2007 A COMPARATIVE STUDY OF THE FLORAS OF CHINA AND CANADA Zhiyao Su College of Forestry, South China Agricultural University, Guangzhou 510642 - P. R. China J. Hugo Cota-Sánchez Department of Biology, University of Saskatchewan, Saskatoon, SK S7N 5E2 - Canada E-mail: [email protected] ABSTRACT at the generic and specific levels that are correlated with more recent geological and In this study, we investigated the floristic climatic variations and ecoenvironment relationships between China and Canada diversity, resulting in differences in floristic based on comparative analysis of their composition. Overall, western and eastern spermatophyte floras. Floristic lists were Canada have a similar number of shared compiled from standard floras and then genera, which suggests multiple migration subjected to cluster analysis using UPGMA events of floristic elements via the Atlantic and NMS ordination methods. Our data and Pacific connections and corridors that demonstrate that the Chinese spermatophyte existed in past geological times. flora is considerably more diverse than that of Canada and that the taxonomic richness Keywords: China, Canada, floristic rela- of seed plants in the floras of China and tionships, taxonomic richness, shared taxa, Canada shows significant variation at the intercontinental disjunction. specific and generic levels. China contains 272 families, 3 318 genera, and 27 078 RESUMEN species (after taxonomic standardization), whereas the spermatophyte flora of Canada En este estudio se investigaron la relaciones includes 145 families, 947 genera, and florísticas entre Canadá y China basados 4 616 species. The results indicate that out en análisis comparativos de las floras de of 553 genera shared by the Chinese and espermatofitas de ambos países. -

Haustorium Initiation and Early Development
Chapter 4 1 Haustorium Initiation and Early 2 Development 3 Pradeepa C.G. Bandaranayake and John I. Yoder 4 4.1 Introduction 5 The ability to develop invasive haustoria is the key feature of parasitic angiosperms. 6 The haustorium attaches the parasite to the host, penetrates the host while keeping 7 its own tissues intact, develops a vascular continuity between the host and parasite 8 and ultimately provides the conduit through which host and parasite materials flow. 9 The ability to make haustoria distinguishes parasitic from non-parasitic plants; 10 indeed, ‘the haustorium embodies the very idea of parasitism’ (Kuijt 1969). 11 This chapter reviews the initiation and pre-attachment development of terminal 12 and lateral haustoria in parasitic Orobanchaceae. Haustoria have been described for 13 many genera of Orobanchaceae, but their initiation and development has been 14 investigated in a relatively small number of species. Most of these studies have 15 investigated the development of terminal haustoria in the weedy species Striga 16 asiatica (Saunders 1933; William 1961; Nickrent et al. 1979; Keyes et al. 2007), 17 S. hermonthica (Okonkwo 1966; Olivier et al. 1991), S. gesnerioides (Okonkwo 18 and Nwoke 1978), Phelipanche aegyptiaca (syn. Orobanche aegyptiaca) and 19 O. cumana (Joel and Losner-Goshen 1994; Zhou et al. 2004) and Alectra vogelii 20 (Nwoke and Okonkwo 1978; Visser et al. 1990). Studies in the development of 21 lateral haustoria have primarily focussed on three facultative species: Agalinis 22 purpurea (Riopel and Musselman 1979; Baird and Riopel 1984), Castilleja exserta 23 (previously known as Orthocarpus purpurascens) (Atsatt et al. -

Indian Paintbrush CASTILLEJA SPECIES
PROPAGATION PROTOCOL FOR Indian Paintbrush CASTILLEJA SPECIES | Tara Luna 62 NATIVEPLANTS | SPRING 2005 Indian paintbrush (Castilleja spp. Michx. [Scrophulariaceae]) is a vibrant, beautiful genus of annual, biennial, and perennial wildflowers that are found exclusively in North America. The majority of species grow in the West, but a few species occur in the central portion of the US. There are more than 150 species and many freely hybridize with one another in areas where their ranges overlap. They are found in a wide range of habitats, ranging from low elevation wetlands and riparian areas to dry grasslands, steppe-shrub communities, and rocky slopes to mid- to high eleva- tion mountain meadows and slopes. The inflorescence is a short or elongate terminal spike bearing tubular-shaped Iflowers that are subtended by numerous colorful bracts. Indian paintbrush is appro- priately named as the bracts graduate in color from green leafy stems to the brightly colored tops of the inflorescence, thus giving the appearance that the tops of the plants have been dipped in paint. Both insects and hummingbirds are attracted to these plants and serve as pollinators. The flower and bract color, even within a single species, can range wildly across the color palette from rich reds, scarlet, and fuchsia to orange, salmon, pink, yellow, and cream. It is not unusual to find a single flower with up to 3 contrasting colors on the showy bracts. Because the floral bracts make up most of the color, they tend to remain showy for several weeks through the growing season. The rich, brilliant, pro- longed color of these species is one reason why they are some of the most desired native species for the home landscape, yet they are not widely available for sale as container plants because of their interesting biology.