THE CORE SVIN Newsletter December 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Congressional Record United States Th of America PROCEEDINGS and DEBATES of the 104 CONGRESS, FIRST SESSION
E PL UR UM IB N U U S Congressional Record United States th of America PROCEEDINGS AND DEBATES OF THE 104 CONGRESS, FIRST SESSION Vol. 141 WASHINGTON, FRIDAY, APRIL 7, 1995 No. 65 House of Representatives The House met at 11 a.m. and was PLEDGE OF ALLEGIANCE DESIGNATING THE HONORABLE called to order by the Speaker pro tem- The SPEAKER pro tempore. Will the FRANK WOLF AS SPEAKER PRO pore [Mr. BURTON of Indiana]. TEMPORE TO SIGN ENROLLED gentleman from New York [Mr. SOLO- BILLS AND JOINT RESOLUTIONS f MON] come forward and lead the House in the Pledge of Allegiance. THROUGH MAY 1, 1995 DESIGNATION OF SPEAKER PRO Mr. SOLOMON led the Pledge of Alle- The SPEAKER pro tempore laid be- TEMPORE giance as follows: fore the House the following commu- The SPEAKER pro tempore laid be- I pledge allegiance to the Flag of the nication from the Speaker of the House fore the House the following commu- United States of America, and to the Repub- of Representatives: nication from the Speaker. lic for which it stands, one nation under God, WASHINGTON, DC, indivisible, with liberty and justice for all. April 7, 1995. WASHINGTON, DC, I hereby designate the Honorable FRANK R. April 7, 1995. f WOLF to act as Speaker pro tempore to sign I hereby designate the Honorable DAN BUR- enrolled bills and joint resolutions through TON to act as Speaker pro tempore on this MESSAGE FROM THE SENATE May 1, 1995. day. NEWT GINGRICH, NEWT GINGRICH, A message from the Senate by Mr. Speaker of the House of Representatives. -

Dragon Magazine
DRAGON 1 Publisher: Mike Cook Editor-in-Chief: Kim Mohan Shorter and stronger Editorial staff: Marilyn Favaro Roger Raupp If this isnt one of the first places you Patrick L. Price turn to when a new issue comes out, you Mary Kirchoff may have already noticed that TSR, Inc. Roger Moore Vol. VIII, No. 2 August 1983 Business manager: Mary Parkinson has a new name shorter and more Office staff: Sharon Walton accurate, since TSR is more than a SPECIAL ATTRACTION Mary Cossman hobby-gaming company. The name Layout designer: Kristine L. Bartyzel change is the most immediately visible The DRAGON® magazine index . 45 Contributing editor: Ed Greenwood effect of several changes the company has Covering more than seven years National advertising representative: undergone lately. in the space of six pages Robert Dewey To the limit of this space, heres some 1409 Pebblecreek Glenview IL 60025 information about the changes, mostly Phone (312)998-6237 expressed in terms of how I think they OTHER FEATURES will affect the audience we reach. For a This issues contributing artists: specific answer to that, see the notice Clyde Caldwell Phil Foglio across the bottom of page 4: Ares maga- The ecology of the beholder . 6 Roger Raupp Mary Hanson- Jeff Easley Roberts zine and DRAGON® magazine are going The Nine Hells, Part II . 22 Dave Trampier Edward B. Wagner to stay out of each others turf from now From Malbolge through Nessus Larry Elmore on, giving the readers of each magazine more of what they read it for. Saved by the cavalry! . 56 DRAGON Magazine (ISSN 0279-6848) is pub- I mention that change here as an lished monthly for a subscription price of $24 per example of what has happened, some- Army in BOOT HILL® game terms year by Dragon Publishing, a division of TSR, Inc. -

Growing a Modern VICTORY GARDEN Everyone Is Looking for Ways They Can Help During the Current COVID-19 Outbreak
Cornell Cooperative Extension April 2020 of Jefferson County Growing a Modern VICTORY GARDEN Everyone is looking for ways they can help during the current COVID-19 outbreak. Planting a garden can be one way for your family to get healthy, fresh vegetables, save money, potentially help others through food donations, get exercise, and relieve stress. Consider these historic facts on Victory Gardens: • World War I: In 1917 home gardeners mobilized and the first Victory Gardens were planted. In 1918, more than 5.2 million gardens were INSIDE THIS GUIDE cultivated. Page 2. No Room for a Garden? Never Fear, Container Gardens are • World War II: In 1944, an estimated 20 million Victory Gardens Here! produced 8 million tons of food. This was 40% of all the fruits and Page 4. Traditional Vegetable Gardens, i.e. Gardening in the vegetables consumed in the U.S. Earth The current COVID-19 situation Page 5. Cool and Warm Season has sparked renewed interest in Vegetables- AKA, When Can I plant? food gardening. Many people are considering vegetable Page 8. Vegetable Transplants gardens as a way to increase Page 9. Everybody Loves Tomatoes! personal food security. This Page 11. Backyard Fruit Production- spring will see first time Some Things to Consider gardens, expansion of current Page 13. Fruits and Nuts You Can Grow in Northern New York gardens, and the re-start of long Page 14. Water, Weeds and Woes dormant food gardens. Page 15. Resources For those current and past Attachment: Cornell Recommended gardeners I don’t expect this information will tell you more than you Vegetable Varieties for New already know, although you may pick up some new tips. -

Information to Users
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the qualify of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form atthe back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA .313/761-4700 800/521-0600 Order Number 00-12,166 The use of leisure and its relation to social stratification Clarke, Alfred Carpenter, Ph.D. Ohio State University, 1955 UMI 300 N. -

Selected List of Vegetable Varieties for Gardeners in New York State
Selected List of Vegetable Varieties for Gardeners in New York State 2021 (Revised 11/20) Vegetable varieties listed in this report should be well adapted for New York State community, school and home gardens. It is recognized that varieties not listed here may be satisfactory or even perform better under certain conditions. We encourage gardens to rate the varieties at: vegvariety.cce.cornell.edu Visit our Vegetable Varieties for Gardeners in New York State website for detailed descriptions and some seed sources of more than 7,000 vegetable varieties including those listed in this report. Compare varieties, read ratings and reviews by fellow gardeners, and offer your own observations of which varieties perform best in your garden. Note: Future reports will be largely based on variety ratings from this site. vegvariety.cce.cornell.edu Cornell Garden-Based Learning Horticulture School of Integrative Plant Sciences Cornell University Ithaca, NY 14853-0327 www.gardening.cornell.edu Selected List of Vegetable Varieties for Gardeners in NYS – A. Helmholdt – page 2 of 8 Key to Notation At least 4 NY gardeners’ ratings averaging # New varieties to this year’s list * >3.5 stars at: vegvariety.cce.cornell.edu Downy mildew resistant or A Alternaria stem resistant DM tolerant Tolerance to Bean Common Mosaic Powdery mildew resistant or BCMV PM Virus BV1 & NY15 tolerant Bacterial leaf spot resistant strains 1, 2, BLS R Rust resistant and 3 are noted CTV Tolerance to citrus tristeza virus SE Sugar enhanced F Fusarium resistant SH2 Supersweet LBR Late -

SW Monthly Onlinecover
Werk Thoroughbred Consultants, Inc. presents WTC’s STAKES & MAIDEN WINNERS MONTHLY October, 2009 Featuring a monthly recap of the winners of unrestricted stakes races in North America and Europe, which includes our WTC “Best of Sale” selections and Mating Recommendations. Also included is a list of all maiden winners for the month sorted by sire. Published by Werk Thoroughbred Consultants, Inc. PO Box 1926, Fremont, CA 94538-0192 (510) 490-1111 / (510) 490-4499 (fax) www.werkhorse.com email: [email protected] Copyright 2009 WERK THOROUGHBRED CONSULTANTS MATINGS and BEST OF SALE STAKES WINNERS (Domestic Stakes $25,000 and up · October 1 through October 31, 2009) NORTH AMERICAN GRADED STAKES RESULTS Grade 1 Races SHADWELL TURF MILE S. ( GI ), KEE, $600,000, 3YO/UP, 1MT, 10-10. — COURT VISION, c, 4, Gulch--Weekend Storm, by Storm Bird. WTC “BEST OF SALE” – KEESEP06 - $180,000 – 2.0 STARS – A NICK ($350,000 2yo 2007 FTFFEB.). O-IEAH Stables and Resolute Group Stables, B-W. S. Farish &Kilroy Thoroughbred Partnership (KY), $360,000. — Karelian, g, 7, Bertrando--Leaning Tower, by Theatrical (IRE). WTC “BEST OF SALE” – KEESEP03 – ($47,000)RNA – 1.5 STARS – C+ NICK O-Green Lantern Stables LLC, B-Green Lantern Stables (KY), $120,000. — Mr. Sidney, h, 5, Storm Cat--Tomisue's Delight, by A.P. Indy. WTC “BEST OF SALE” – KEESEP05 - $3,900,000 – 3.5 STARS – A NICK O-Circle E Racing, B-Hilbert Thoroughbreds (KY), $60,000. JOE HIRSCH TURF CLASSIC INVITATIONAL S. ( GI ), BEL, $600,000, 3YO/UP, 1 1/2MT, 10-3. —INTERPATATION, g, 7, Langfuhr--Idealistic Cause, by Habitony. -
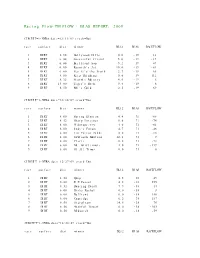
Racing Flow-TM FLOW + BIAS REPORT: 2009
Racing Flow-TM FLOW + BIAS REPORT: 2009 CIRCUIT=1-NYRA date=12/31/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 5.50 Hollywood Hills 0.0 -19 13 2 DIRT 6.00 Successful friend 5.0 -19 -19 3 DIRT 6.00 Brilliant Son 5.2 -19 47 4 DIRT 6.00 Raynick's Jet 10.6 -19 -61 5 DIRT 6.00 Yes It's the Truth 2.7 -19 65 6 DIRT 8.00 Keep Thinking 0.0 -19 -112 7 DIRT 8.32 Storm's Majesty 4.0 -19 6 8 DIRT 13.00 Tiger's Rock 9.4 -19 6 9 DIRT 8.50 Mel's Gold 2.5 -19 69 CIRCUIT=1-NYRA date=12/30/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 8.00 Spring Elusion 4.4 71 -68 2 DIRT 8.32 Sharp Instinct 0.0 71 -74 3 DIRT 6.00 O'Sotopretty 4.0 71 -61 4 DIRT 6.00 Indy's Forum 4.7 71 -46 5 DIRT 6.00 Ten Carrot Nikki 0.0 71 -18 6 DIRT 8.00 Sawtooth Moutain 12.1 71 9 7 DIRT 6.00 Cleric 0.6 71 -73 8 DIRT 6.00 Mt. Glittermore 4.0 71 -119 9 DIRT 6.00 Of All Times 0.0 71 0 CIRCUIT=1-NYRA date=12/27/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 8.50 Quip 4.5 -38 49 2 DIRT 6.00 E Z Passer 4.2 -38 255 3 DIRT 8.32 Dancing Daisy 7.9 -38 14 4 DIRT 6.00 Risky Rachel 0.0 -38 8 5 DIRT 6.00 Kaffiend 0.0 -38 150 6 DIRT 6.00 Capridge 6.2 -38 187 7 DIRT 8.50 Stargleam 14.5 -38 76 8 DIRT 8.50 Wishful Tomcat 0.0 -38 -203 9 DIRT 8.50 Midwatch 0.0 -38 -59 CIRCUIT=1-NYRA date=12/26/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 6.00 Papaleo 7.0 108 129 2 DIRT 6.00 Overcommunication 1.0 108 -72 3 DIRT 6.00 Digger 0.0 108 -211 4 DIRT 6.00 Bryan Kicks 0.0 108 136 5 DIRT 6.00 We Get It 16.8 108 129 6 DIRT 6.00 Yawanna Trust 4.5 108 -21 7 DIRT 6.00 Smarty Karakorum 6.5 108 83 8 DIRT 8.32 Almighty Silver 18.7 108 133 9 DIRT 8.32 Offlee Cool 0.0 108 -60 CIRCUIT=1-NYRA date=12/13/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 8.32 Crafty Bear 3.0 -158 -139 2 DIRT 6.00 Cheers Darling 0.5 -158 61 3 DIRT 6.00 Iberian Gate 3.0 -158 154 4 DIRT 6.00 Pewter 0.5 -158 8 5 DIRT 6.00 Wolfson 6.2 -158 86 6 DIRT 6.00 Mr. -

\0-9\0 and X ... \0-9\0 Grad Nord ... \0-9\0013 ... \0-9\007 Car Chase ... \0-9\1 X 1 Kampf ... \0-9\1, 2, 3
... \0-9\0 and X ... \0-9\0 Grad Nord ... \0-9\0013 ... \0-9\007 Car Chase ... \0-9\1 x 1 Kampf ... \0-9\1, 2, 3 ... \0-9\1,000,000 ... \0-9\10 Pin ... \0-9\10... Knockout! ... \0-9\100 Meter Dash ... \0-9\100 Mile Race ... \0-9\100,000 Pyramid, The ... \0-9\1000 Miglia Volume I - 1927-1933 ... \0-9\1000 Miler ... \0-9\1000 Miler v2.0 ... \0-9\1000 Miles ... \0-9\10000 Meters ... \0-9\10-Pin Bowling ... \0-9\10th Frame_001 ... \0-9\10th Frame_002 ... \0-9\1-3-5-7 ... \0-9\14-15 Puzzle, The ... \0-9\15 Pietnastka ... \0-9\15 Solitaire ... \0-9\15-Puzzle, The ... \0-9\17 und 04 ... \0-9\17 und 4 ... \0-9\17+4_001 ... \0-9\17+4_002 ... \0-9\17+4_003 ... \0-9\17+4_004 ... \0-9\1789 ... \0-9\18 Uhren ... \0-9\180 ... \0-9\19 Part One - Boot Camp ... \0-9\1942_001 ... \0-9\1942_002 ... \0-9\1942_003 ... \0-9\1943 - One Year After ... \0-9\1943 - The Battle of Midway ... \0-9\1944 ... \0-9\1948 ... \0-9\1985 ... \0-9\1985 - The Day After ... \0-9\1991 World Cup Knockout, The ... \0-9\1994 - Ten Years After ... \0-9\1st Division Manager ... \0-9\2 Worms War ... \0-9\20 Tons ... \0-9\20.000 Meilen unter dem Meer ... \0-9\2001 ... \0-9\2010 ... \0-9\21 ... \0-9\2112 - The Battle for Planet Earth ... \0-9\221B Baker Street ... \0-9\23 Matches .. -

NPRC) VIP List, 2009
Description of document: National Archives National Personnel Records Center (NPRC) VIP list, 2009 Requested date: December 2007 Released date: March 2008 Posted date: 04-January-2010 Source of document: National Personnel Records Center Military Personnel Records 9700 Page Avenue St. Louis, MO 63132-5100 Note: NPRC staff has compiled a list of prominent persons whose military records files they hold. They call this their VIP Listing. You can ask for a copy of any of these files simply by submitting a Freedom of Information Act request to the address above. The governmentattic.org web site (“the site”) is noncommercial and free to the public. The site and materials made available on the site, such as this file, are for reference only. The governmentattic.org web site and its principals have made every effort to make this information as complete and as accurate as possible, however, there may be mistakes and omissions, both typographical and in content. The governmentattic.org web site and its principals shall have neither liability nor responsibility to any person or entity with respect to any loss or damage caused, or alleged to have been caused, directly or indirectly, by the information provided on the governmentattic.org web site or in this file. The public records published on the site were obtained from government agencies using proper legal channels. Each document is identified as to the source. Any concerns about the contents of the site should be directed to the agency originating the document in question. GovernmentAttic.org is not responsible for the contents of documents published on the website. -

Canada: the Next Country of Europe
GENERAL RICK HILLIER ON THE DECADE OF DARKNESS March–April 2007 Canada: The Next Country of Europe EU AMBASSADOR DORIAN PRINCE WANTS A FREE TRADE AGREEMENT WITHIN FOUR YEARS. WITH FULL MOBILITY RIGHTS. A chat with Britain’s new high commissioner Burkas and mini-skirts in Istanbul Ghana at 50: A struggle for democracy in Africa ESTABLISHED 1989 CDN $5.95 PM 40957514 Impressive setting … outstanding service … memorable events Consider holding your next event in an exciting cultural venue—the Canada Science and Technology Museum. Home to Canada’s most comprehensive science and technology collection, the Museum’s exhibition halls provide distinctive spaces for banquets, receptions or cocktail parties. In addition, dynamic, trained staff is available to offer your guests guided tours of the exhibitions, 1867 St Laurent Boulevard behind-the-scenes collection tours, astronomy programs, family-oriented activities, or workshops Ottawa, Ontario for children. sciencetech.technomuses.ca For more information, contact the facilities rental manager, Suzanne Beaulne at 613.991.6091, or by email, [email protected]. GENERAL RICK HILLIER ON THE DECADE OF DARKNESS March–April 2007 Canada: The Next Country of Europe EU AMBASSADOR DORIAN PRINCE WANTS A FREE TRADE AGREEMENT WITHIN FOUR YEARS. WITH FULL MOBILITY RIGHTS. A chat with Britain’s new high commissioner Burkas and mini-skirts in Istanbul Ghana at 50: A struggle for democracy in Africa ESTABLISHED 1989 CDN $5.95 The World in Canada PM 40957514 Volume 18, Number 1 PUBLISHER Neil Reynolds ASSOCIATE PUBLISHER Table of Donna Jacobs EDITOR CONTENTS Jennifer Campbell ART DIRECTOR Paul Cavanaugh CONTRIBUTING EDITORS Daniel Drolet George Abraham Charles Enman CULTURE EDITOR Margo Roston COPY EDITOR DIPLOMATICA| Roger Bird Verbatim: An excerpt from the Decade of Darkness speech . -

Protocol for the Modern Diplomat, and Make a Point of Adopting and Practicing This Art and Craft During Your Overseas Assignment
Mission Statement “The Foreign Service Institute develops the men and women our nation requires to fulfill our leadership role in world affairs and to defend U.S. interests.” About FSI Established in 1947, the Foreign Service Institute is the United States Government’s primary training institution for employees of the U.S. foreign affairs community, preparing American diplomats and other professionals to advance U.S. foreign affairs interests overseas and in Washington. FSI provides more than 600 courses – to include training in some 70 foreign languages, as well as in leadership, management, professional tradecraft, area studies, and applied information technology skills – to some 100,000 students a year, drawn from the Department of State and more than 40 other government agencies and military service branches. FSI provides support to all U.S. Government employees involved in foreign affairs, from State Department entry-level specialists and generalists to newly-assigned Ambassadors, and to our Foreign Service National colleagues who assist U.S. efforts at some 270 posts abroad. i Table of Contents Introduction ..................................................................................................................................... 1 Protocol In Brief ............................................................................................................................. 2 International Culture ....................................................................................................................... 2 Addressing -

Free Card Games Solitaire Download
Free card games solitaire download Solitaire fans will love Free Solitaire's collection of free solitaire card games for Windows. It includes 12 solitaire variations ranging from Diplomat. Free Solitaire latest version: Twelve free solitaire games in one pack. Free Solitaire is the logical evolution of the good old solitaire game we all used to. Download card game Klondike solitaire and play offline for absolutely free! Maximize it to your entire computer screen to play card games as large as you like. Download Freecell Solitaire. Like to play Totally free; Share with everyone you know; Change the card game window size; Play without the need to be online. Play solitaire for free. No download or registration needed. Solitaire♤ Spider中 Mahjong. About. New Game. Options. How many cards to turn at once? Turn 1. If you like solitaire, you're going to love Solitaire Suite, the #1 pack of card games available for Android. With different games, including popular classics. Solitaire by MobilityWare is the original Solitaire game for Android devices! Join over million users playing our Solitaire for Android! Our version of Solitaire. Looking for something exciting and classic? you can now have for Free one of the best Solitaire games available on the market. Just grab your Android device. Solitaire Card Games Free is available to download for free on your Android Phone. Free Solitaire Card Games is among one of the most. Solitaire Games - Free Download! All Solitaire Games are % Free Full Version. No payments, No time limits, No trials, No ads. New game added every 3. Play Klondike, Spider, Freecell, Pyramid, and TriPeaks.