Betaxolol Ophthalmic Solution As Alternative Treatment for Patients with Timolol Allergy: a Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Topical Beta-Adrenoceptor Blocking Agents on Pulsatile Ocular Blood Flow
THE EFFECT OF TOPICAL BETA-ADRENOCEPTOR BLOCKING AGENTS ON PULSATILE OCULAR BLOOD FLOW C. D. MORSMAN, M. E. BOSEM, M. LUSKY and R. N. WEINREB San Diego, California SUMMARY factors (e.g. hypertension, diabetes, peripheral Thirty-three ocular hypertensive patients (21 with vascular disease and vasospasm) to the vascular primary open angle glaucoma and 12 glaucoma system suggest that blood flow in the optic nerve suspects) were randomly assigned to receive either head and retina may be altered in glaucoma.4 Of the timolol, levobunolol or betaxolol in one eye. Pulsatile various vascular beds within the posterior segment, ocular blood flow (POBF) was measured before the choroidal circulation is of particular interest since treatment (baseline) and 2 hours after drop adminis it provides the major contribution to the blood flow tration. After 1 week of regular twice-daily dosage, of the optic nerve at the level of the lamina cribrosa.5 POBF was measured again both immediately before Beta-2 adrenergic receptors have been demon and 2 hours after drop instillation. All measurements strated in human optic nerve,6 as well as in choroidal were made by an investigator masked to treatment. and retinal blood vessels.7 Blockade of these POBF increased by 11% (p = 0.09) at week 0 after receptors can cause vasoconstrictionS which could levobunolol administration, and by 22% (p = 0.20) at adversely affect visual function if adequate concen week 1 before drop administration compared with trations of the drug diffused into the posterior baseline. It dropped by 23% and 25% (p = 0.04 and segment of the eye or were absorbed systemically. -

Use of Emulsions for Intra- and Periocular Injection
(19) & (11) EP 1 611 879 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/107 (2006.01) 12.08.2009 Bulletin 2009/33 (21) Application number: 04291684.1 (22) Date of filing: 02.07.2004 (54) Use of emulsions for intra- and periocular injection Verwendung von Emulsionen zur intra- und periocularen Injection Utilisation des émulsions pour injection intra- et périoculaire. (84) Designated Contracting States: (74) Representative: de Mareüil-Villette, Caroline et al AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Cabinet Plasseraud HU IE IT LI LU MC NL PL PT RO SE SI SK TR 52 rue de la Victoire 75440 Paris Cedex 09 (FR) (43) Date of publication of application: 04.01.2006 Bulletin 2006/01 (56) References cited: EP-A- 0 521 799 EP-A- 1 020 194 (73) Proprietors: WO-A-02/09667 WO-A-93/18852 • Novagali Pharma S.A. WO-A-94/05298 WO-A-03/053405 91000 Evry (FR) US-A- 5 632 984 • CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS) • KLANG S H ET AL: "PHYSICOCHEMICAL 75016 Paris (FR) CHARACTERIAZATION AND ACUTE TOXICITY • INSTITUT NATIONAL DE LA SANTE ET DE LA EVALUATION OF A POSITIVELY-CHARGED RECHERCHE MEDICALE (INSERM) SUBMICRON EMULSION VEHICLE" JOURNAL 75013 Paris (FR) OF PHARMACY AND PHARMACOLOGY, • YISSUM RESEARCH DEVELOPMENT COMPANY LONDON, GB, vol. 46, no. 12, December 1994 OF THE HEBREW UNIVERSITY OF JERUSALEM (1994-12), pages 986-993, XP008005426 ISSN: 91390 Jerusalem (IL) 0022-3573 • KLANG S ET AL: "INFLUENCE OF EMULSION (72) Inventors: DROPLET SURFACE CHARGE ON • Rabinovich-Guilatt, Laura INDOMETHACIN OCULAR TISSUE 75015 Paris (FR) DISTRIBUTION" PHARMACEUTICAL • De Kozak, Yvonne DEVELOPMENT AND TECHNOLOGY, NEW 75013 Paris (FR) YORK, NY, US, vol. -

Fluid Ophthalmic Composition Based on Lipid Microparticles Containing at Least One Active Principle
Europaisches Patentamt J European Patent Office Office europden des brevets (11) Publication number : 0 437 368 A1 EUROPEAN PATENT APPLICATION (21) Application number: 91300181.4 ® int. ci.5 : A61K 9/06, A61K 9/16 @ Date of filing : 10.01.91 © Priority : 12.01.90 FR 9000340 (72) Inventor : Rozier, Annouk 23 Bd Lafayette F-63000 Clermont-Ferrand (FR) @ Date of publication of application : 17.07.91 Bulletin 91/29 74) Representative : Hesketh, Alan, Dr. et al European Patent Department Merck & Co., @ Designated Contracting States : Inc. Tertings Park Eastwick Road CH DE FR GB IT LI NL Harlow Essex, CM20 2QR (GB) © Applicant : LABORATOIRES MERCK, SHARP & DOHME-CHIBRET 3, Avenue Hoche F-75008 Paris (FR) (S) Fluid ophthalmic composition based on lipid microparticles containing at least one active principle. (57) There is described a fluid ophthalmic composition which comprises a suspension in a fluid dispersant medium of lipid microparticles containing at least one active principle. The composition enables improved availability of the active principle to be obtained as a result of high intraocular levels. 00 <0 CO Q. UJ Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 437 368 A1 FLUID OPHTHALMIC COMPOSITION BASED ON LIPID MICROPARTICLES CONTAINING AT LEAST ONE ACTIVE PRINCIPLE The present invention relates to a fluid ophthalmic composition. Many ophthalmic compositions are currently available in liquid or solid form, but none of them is, in fact, completely satisfactory. In effect, liquid ophthalmic compositions, although easy to use, have some drawbacks ; in particular, it is 5 difficult to obtain a sustained or delayed action of the active principle which they contain. -
Guidance for the Format and Content of the Protocol of Non-Interventional
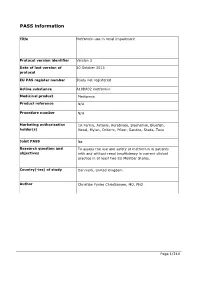
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Canine Red Eye Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO
PEER REVIEWED Clinical Approach to the CANINE RED EYE Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO he acute red eye is a common clinical challenge for tion of the deep episcleral vessels, and is characterized general practitioners. Redness is the hallmark of by straight and immobile episcleral vessels, which run Tocular inflammation; it is a nonspecific sign related 90° to the limbus. Episcleral injection is an external to a number of underlying diseases and degree of redness sign of intraocular disease, such as anterior uveitis and may not reflect the severity of the ocular problem. glaucoma (Figures 3 and 4). Occasionally, episcleral Proper evaluation of the red eye depends on effective injection may occur in diseases of the sclera, such as and efficient diagnosis of the underlying ocular disease in episcleritis or scleritis.1 order to save the eye’s vision and the eye itself.1,2 • Corneal Neovascularization » Superficial: Long, branching corneal vessels; may be SOURCE OF REDNESS seen with superficial ulcerative (Figure 5) or nonul- The conjunctiva has small, fine, tortuous and movable vessels cerative keratitis (Figure 6) that help distinguish conjunctival inflammation from deeper » Focal deep: Straight, nonbranching corneal vessels; inflammation (see Ocular Redness algorithm, page 16). indicates a deep corneal keratitis • Conjunctival hyperemia presents with redness and » 360° deep: Corneal vessels in a 360° pattern around congestion of the conjunctival blood vessels, making the limbus; should arouse concern that glaucoma or them appear more prominent, and is associated with uveitis (Figure 4) is present1,2 extraocular disease, such as conjunctivitis (Figure 1). -

NEW CONCEPTS in GLAUCOMA CARE TREATMENT Proceedings of the Fifteenth Annual Meeting & of the Optometric Glaucoma Society
NEW CONCEPTS IN GLAUCOMA CARE TREATMENT Proceedings of the Fifteenth Annual Meeting & of the Optometric Glaucoma Society INSIDE: • Virtual Reality Uses in Glaucoma • Questions Glaucoma Patients Ask • Pathogenesis of Glaucoma • Glaucoma Progression • Real-Time Aqueous Humor Outfl ow Imaginging APRIL 2017 REVIEW OF OPTOMETRY/APRIL 2017 1 0217_OGS_ja_3.22.indd 1 3/24/17 3:36 PM ro0417ogs_vyzulta.indd 1 3/20/17 1:57 PM NEW CONCEPTS IN GLAUCOMA CARE TREATMENT TABLE OF CONTENTS INTRODUCTORY REMARKS The 15th Annual Scientifi c Meeting of the Optometric Glaucoma Society (OGS), held Nov. 15 and 16, 2016, in Anaheim, Calif., brought 3 together some of the country’s top luminaries INTRODUCTORY REMARKS in the areas of glaucoma diagnosis, treatment, Highlights From the Annual Scientifi c assessment, and management. These individu- Meeting als shared groundbreaking research and the BY MURRAY FINGERET, OD latest clinical knowledge about glaucoma—con- sidered to be the top global eye burden by the World Health Organization. 4 Kicking things off in the President’s Lecture, PRESIDENT’S LECTURE Felipe A. Medeiros, MD, PhD, highlighted potential clinical applications for More Than a Video Game: Virtual virtual reality devices. These devices, being tested in simulation laboratories, Reality and Its Uses in Glaucoma could one day assist clinicians in assessing patients at risk for glaucoma and BY FELIPE A. MEDEIROS, MD, PHD in danger of falls and motor vehicle accidents due to visual fi eld loss. Make no mistake: These cutting-edge tools are not your techie’s virtual reality. Dr. Medeiros, in a separate lecture about glaucoma progression, unveiled 6 an innovative metric developed by his research group to measure functional PATIENT CARE and structural vision loss in glaucoma patients. -

Efficacy and Safety of the Fixed Combinations of Tafluprost/Timolol
www.nature.com/scientificreports OPEN Efcacy and safety of the fxed combinations of tafuprost/timolol and latanoprost/carteolol Received: 4 February 2019 Masahiro Fuwa, Atsushi Shimazaki, Masafumi Mieda, Naoko Yamashita, Takahiro Akaishi, Accepted: 7 May 2019 Takazumi Taniguchi & Masatomo Kato Published: xx xx xxxx In this study, we made a comparative efcacy and safety assessment of two diferent fxed combinations of drugs, viz., tafuprost/timolol (TAF/TIM) and latanoprost/carteolol (LAT/CAR), by determining their efects on intraocular pressure (IOP) in ocular normotensive monkeys and examining their toxic efects on ocular surface using human corneal epithelial cells. TAF/TIM was found to be more efective in lowering IOP for a longer duration compared to LAT/CAR. We found that the diference in the intensity of IOP-lowering efect was because of the diferences in the strength of timolol compared with that of carteolol as a beta-adrenergic antagonist and strength of tafuprost compared with that of latanoprost as a prostaglandin analogue. In addition, TAF/TIM showed much less cytotoxic efects compared to LAT/CAR on the human corneal epithelial cells. Our fndings showed that TAF/TIM is better than LAT/CAR with regard to the IOP-lowering efect in monkeys and toxicity on ocular surface. Glaucoma is a neurodegenerative disease of the eyes characterised by selective retinal ganglion cell loss, fol- lowed by progressive defects in visual feld, resulting in the principal cause of irreversible blindness worldwide1–4. Elevated intraocular pressure (IOP) is an important contributor for the progression of glaucoma, for which the current treatment primarily involves IOP reduction1,5–8. -

Antiglaucoma Compositions Containing Combinations of Alpha-2 Agonists and Beta-Blockers
~" ' MM II II II MM II II II Ml II II I II J European Patent Office _ _ _ © Publication number: 0 365 662 B1 Office europeen* des.. brevets , © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 09.03.94 © Int. CI.5: A61 K 31/47 © Application number: 89905874.7 @ Date of filing: 26.04.89 © International application number: PCT/US89/01994 © International publication number: WO 89/10126 (02.11.89 89/26) (54) ANTIGLAUCOMA COMPOSITIONS CONTAINING COMBINATIONS OF ALPHA-2 AGONISTS AND BETA-BLOCKERS. ® Priority: 26.04.88 US 186504 1075-1078 @ Date of publication of application: J. of Cardiovascular Pharmacology, vol. 2, 02.05.90 Bulletin 90/18 suppl. 1, 1980, S. 21-28 © Publication of the grant of the patent: © Proprietor: ALCON LABORATORIES INC 09.03.94 Bulletin 94/10 6201 South Freeway Ft. Worth, TX 76134(US) © Designated Contracting States: AT BE CH DE FR GB IT LI LU NL SE @ Inventor: DE SANTIS, Louis, M. 2316 Wlnton Terrace West © References cited: Fort Worth, TX 761 09(US) US-A- 4 455 317 US-A- 4 515 800 © Representative: Jump, Timothy John Simon et AM A DRUG EVALUATION, 2nd ed., 1973; al "Agent used to treat Glaucoma", pp. 675-686. Venner Shipley & Co. 20 Little Britain GLAUCOMA, vol. 1, no. 1, February 1979; London EC1A 7DH (GB) 00 S.SUGAR, pp. 9-15. CM CO Ophthalmology, vol. 96, no. 1, 1989, p. 3-7 CO m Ophthalmology, vol. 98, no. 7, 1991, p. CO 00 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Glaucoma Medical Treatment: Philosophy, Principles and Practice
Glaucoma medical CLIVE MIGDAL treatment: philosophy, principles and practice Abstract assessment of these parameters. Indeed There have been numerous recent advances in compounds are under evaluation that affect the the management of glaucoma, not least the function of the optic nerve (via improved blood development of new drugs to help manage supply or improved neuronal cell physiology) raised intraocular pressure. In addition, the but may or may not lower lOP. It may even be concepts of improving blood flow to the optic possible in the future to therapeutically alter the nerve head and neuroprotection are currently human genome, genetically deliver provoking considerable interest. This article neuroprotective substances or aid regeneration considers the aims and philosophy of of the optic nerve axons. glaucoma drug therapy, summarises some of The main aim of glaucoma therapy must still the basic facts and principles of modem be the preservation of visual function. At the glaucoma medications, and suggests a same time, the therapy should not have adverse practical approach to the choice of therapy. side effects and should not affect the quality of life of the patient (by causing either side effects Key words Blood flow, Intraocular pressure, or inconvenience and disruption of daily Neuroprotection, Primary open angle glaucoma, Topical medications lifestyle). The cost of the therapy, both direct and indirect, must also be taken into consideration.s Currently, typical glaucoma management Philosophy consists of lowering the lOP to a satisfactory Primary open-angle glaucoma is a complex and safe target leve1.6 To determine the success disease for which a number of risk factors have of this treatment, the patient must be followed been identified, including intraocular pressure, long-term with routine assessment of lOP, discs age, race and family history.l,2 Due to our and fields to exclude progressive damage. -

PHRP March 2015
March 2015; Vol. 25(2):e2521518 doi: http://dx.doi.org/10.17061/phrp2521518 www.phrp.com.au Research Manual versus automated coding of free-text self-reported medication data in the 45 and Up Study: a validation study Danijela Gnjidica,b,i, Sallie-Anne Pearsona,c, Sarah N Hilmerb,d, Jim Basilakise, Andrea L Schaffera, Fiona M Blythb,f,g and Emily Banksg,h, on behalf of the High Risk Prescribing Investigators a Faculty of Pharmacy, University of Sydney, NSW, Australia b Sydney Medical School, University of Sydney, NSW, Australia c Sydney School of Public Health, University of Sydney, NSW, Australia d Royal North Shore Hospital and Kolling Institute of Medical Research, Sydney, NSW, Australia e School of Computing, Engineering and Mathematics, University of Western Sydney, NSW, Australia f Centre for Education and Research on Ageing (CERA), Concord Hospital, Sydney, NSW, Australia g The Sax Institute, Sydney, NSW, Australia h National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT i Corresponding author: [email protected] Article history Abstract Publication date: March 2015 Background: Increasingly, automated methods are being used to code free- Citation: Gnjidic D, Pearson S, Hilmer S, text medication data, but evidence on the validity of these methods is limited. Basilakis J, Schaffer AL, Blyth FM, Banks E. To examine the accuracy of automated coding of previously keyed Manual versus automated coding of free-text Aim: in free-text medication data compared with manual coding of original self-reported medication data in the 45 and Up Study: a validation study. Public Health handwritten free-text responses (the ‘gold standard’). -

Betaxolol 0.5% Eye Drops
Package leaflet: Information for the patient BETAXOLOL 0.5% EYE DROPS Betaxolol (as hydrochloride) Read all of this leaflet carefully before you start using this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or pharmacist. • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. • If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. • Your medicine, Betaxolol 0.5% Eye Drops will be referred to as Betaxolol in this leaflet. What is in this leaflet 1. What Betaxolol is and what it is used for 2. What you need to know before you use Betaxolol 3. How to use Betaxolol 4. Possible side effects 5. How to store Betaxolol 6. Contents of the pack and other information 1. What Betaxolol is and what it is used for The active ingredient betaxolol belongs to a group of medicines called beta-blockers. Betaxolol is used to treat raised pressure of eye (intraocular pressure) which occurs in various conditions including glaucoma and ocular hypertension (high pressure in the eye) by reducing the fluid pressure in your eye(s). 2. What you need to know before you use Betaxolol Do not use Betaxolol • if you are allergic (hypersensitive) to betaxolol hydrochloride or beta-blockers or any of the other ingredients of this medicine (listed in section 6).