Betaxolol 0.5% Eye Drops
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Topical Beta-Adrenoceptor Blocking Agents on Pulsatile Ocular Blood Flow
THE EFFECT OF TOPICAL BETA-ADRENOCEPTOR BLOCKING AGENTS ON PULSATILE OCULAR BLOOD FLOW C. D. MORSMAN, M. E. BOSEM, M. LUSKY and R. N. WEINREB San Diego, California SUMMARY factors (e.g. hypertension, diabetes, peripheral Thirty-three ocular hypertensive patients (21 with vascular disease and vasospasm) to the vascular primary open angle glaucoma and 12 glaucoma system suggest that blood flow in the optic nerve suspects) were randomly assigned to receive either head and retina may be altered in glaucoma.4 Of the timolol, levobunolol or betaxolol in one eye. Pulsatile various vascular beds within the posterior segment, ocular blood flow (POBF) was measured before the choroidal circulation is of particular interest since treatment (baseline) and 2 hours after drop adminis it provides the major contribution to the blood flow tration. After 1 week of regular twice-daily dosage, of the optic nerve at the level of the lamina cribrosa.5 POBF was measured again both immediately before Beta-2 adrenergic receptors have been demon and 2 hours after drop instillation. All measurements strated in human optic nerve,6 as well as in choroidal were made by an investigator masked to treatment. and retinal blood vessels.7 Blockade of these POBF increased by 11% (p = 0.09) at week 0 after receptors can cause vasoconstrictionS which could levobunolol administration, and by 22% (p = 0.20) at adversely affect visual function if adequate concen week 1 before drop administration compared with trations of the drug diffused into the posterior baseline. It dropped by 23% and 25% (p = 0.04 and segment of the eye or were absorbed systemically. -

Use of Emulsions for Intra- and Periocular Injection
(19) & (11) EP 1 611 879 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/107 (2006.01) 12.08.2009 Bulletin 2009/33 (21) Application number: 04291684.1 (22) Date of filing: 02.07.2004 (54) Use of emulsions for intra- and periocular injection Verwendung von Emulsionen zur intra- und periocularen Injection Utilisation des émulsions pour injection intra- et périoculaire. (84) Designated Contracting States: (74) Representative: de Mareüil-Villette, Caroline et al AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Cabinet Plasseraud HU IE IT LI LU MC NL PL PT RO SE SI SK TR 52 rue de la Victoire 75440 Paris Cedex 09 (FR) (43) Date of publication of application: 04.01.2006 Bulletin 2006/01 (56) References cited: EP-A- 0 521 799 EP-A- 1 020 194 (73) Proprietors: WO-A-02/09667 WO-A-93/18852 • Novagali Pharma S.A. WO-A-94/05298 WO-A-03/053405 91000 Evry (FR) US-A- 5 632 984 • CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS) • KLANG S H ET AL: "PHYSICOCHEMICAL 75016 Paris (FR) CHARACTERIAZATION AND ACUTE TOXICITY • INSTITUT NATIONAL DE LA SANTE ET DE LA EVALUATION OF A POSITIVELY-CHARGED RECHERCHE MEDICALE (INSERM) SUBMICRON EMULSION VEHICLE" JOURNAL 75013 Paris (FR) OF PHARMACY AND PHARMACOLOGY, • YISSUM RESEARCH DEVELOPMENT COMPANY LONDON, GB, vol. 46, no. 12, December 1994 OF THE HEBREW UNIVERSITY OF JERUSALEM (1994-12), pages 986-993, XP008005426 ISSN: 91390 Jerusalem (IL) 0022-3573 • KLANG S ET AL: "INFLUENCE OF EMULSION (72) Inventors: DROPLET SURFACE CHARGE ON • Rabinovich-Guilatt, Laura INDOMETHACIN OCULAR TISSUE 75015 Paris (FR) DISTRIBUTION" PHARMACEUTICAL • De Kozak, Yvonne DEVELOPMENT AND TECHNOLOGY, NEW 75013 Paris (FR) YORK, NY, US, vol. -
Guidance for the Format and Content of the Protocol of Non-Interventional
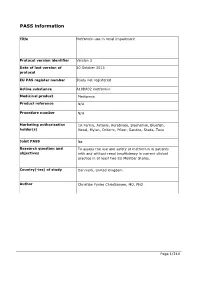
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

Canine Red Eye Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO
PEER REVIEWED Clinical Approach to the CANINE RED EYE Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO he acute red eye is a common clinical challenge for tion of the deep episcleral vessels, and is characterized general practitioners. Redness is the hallmark of by straight and immobile episcleral vessels, which run Tocular inflammation; it is a nonspecific sign related 90° to the limbus. Episcleral injection is an external to a number of underlying diseases and degree of redness sign of intraocular disease, such as anterior uveitis and may not reflect the severity of the ocular problem. glaucoma (Figures 3 and 4). Occasionally, episcleral Proper evaluation of the red eye depends on effective injection may occur in diseases of the sclera, such as and efficient diagnosis of the underlying ocular disease in episcleritis or scleritis.1 order to save the eye’s vision and the eye itself.1,2 • Corneal Neovascularization » Superficial: Long, branching corneal vessels; may be SOURCE OF REDNESS seen with superficial ulcerative (Figure 5) or nonul- The conjunctiva has small, fine, tortuous and movable vessels cerative keratitis (Figure 6) that help distinguish conjunctival inflammation from deeper » Focal deep: Straight, nonbranching corneal vessels; inflammation (see Ocular Redness algorithm, page 16). indicates a deep corneal keratitis • Conjunctival hyperemia presents with redness and » 360° deep: Corneal vessels in a 360° pattern around congestion of the conjunctival blood vessels, making the limbus; should arouse concern that glaucoma or them appear more prominent, and is associated with uveitis (Figure 4) is present1,2 extraocular disease, such as conjunctivitis (Figure 1). -

Antiglaucoma Compositions Containing Combinations of Alpha-2 Agonists and Beta-Blockers
~" ' MM II II II MM II II II Ml II II I II J European Patent Office _ _ _ © Publication number: 0 365 662 B1 Office europeen* des.. brevets , © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 09.03.94 © Int. CI.5: A61 K 31/47 © Application number: 89905874.7 @ Date of filing: 26.04.89 © International application number: PCT/US89/01994 © International publication number: WO 89/10126 (02.11.89 89/26) (54) ANTIGLAUCOMA COMPOSITIONS CONTAINING COMBINATIONS OF ALPHA-2 AGONISTS AND BETA-BLOCKERS. ® Priority: 26.04.88 US 186504 1075-1078 @ Date of publication of application: J. of Cardiovascular Pharmacology, vol. 2, 02.05.90 Bulletin 90/18 suppl. 1, 1980, S. 21-28 © Publication of the grant of the patent: © Proprietor: ALCON LABORATORIES INC 09.03.94 Bulletin 94/10 6201 South Freeway Ft. Worth, TX 76134(US) © Designated Contracting States: AT BE CH DE FR GB IT LI LU NL SE @ Inventor: DE SANTIS, Louis, M. 2316 Wlnton Terrace West © References cited: Fort Worth, TX 761 09(US) US-A- 4 455 317 US-A- 4 515 800 © Representative: Jump, Timothy John Simon et AM A DRUG EVALUATION, 2nd ed., 1973; al "Agent used to treat Glaucoma", pp. 675-686. Venner Shipley & Co. 20 Little Britain GLAUCOMA, vol. 1, no. 1, February 1979; London EC1A 7DH (GB) 00 S.SUGAR, pp. 9-15. CM CO Ophthalmology, vol. 96, no. 1, 1989, p. 3-7 CO m Ophthalmology, vol. 98, no. 7, 1991, p. CO 00 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Glaucoma Medical Treatment: Philosophy, Principles and Practice
Glaucoma medical CLIVE MIGDAL treatment: philosophy, principles and practice Abstract assessment of these parameters. Indeed There have been numerous recent advances in compounds are under evaluation that affect the the management of glaucoma, not least the function of the optic nerve (via improved blood development of new drugs to help manage supply or improved neuronal cell physiology) raised intraocular pressure. In addition, the but may or may not lower lOP. It may even be concepts of improving blood flow to the optic possible in the future to therapeutically alter the nerve head and neuroprotection are currently human genome, genetically deliver provoking considerable interest. This article neuroprotective substances or aid regeneration considers the aims and philosophy of of the optic nerve axons. glaucoma drug therapy, summarises some of The main aim of glaucoma therapy must still the basic facts and principles of modem be the preservation of visual function. At the glaucoma medications, and suggests a same time, the therapy should not have adverse practical approach to the choice of therapy. side effects and should not affect the quality of life of the patient (by causing either side effects Key words Blood flow, Intraocular pressure, or inconvenience and disruption of daily Neuroprotection, Primary open angle glaucoma, Topical medications lifestyle). The cost of the therapy, both direct and indirect, must also be taken into consideration.s Currently, typical glaucoma management Philosophy consists of lowering the lOP to a satisfactory Primary open-angle glaucoma is a complex and safe target leve1.6 To determine the success disease for which a number of risk factors have of this treatment, the patient must be followed been identified, including intraocular pressure, long-term with routine assessment of lOP, discs age, race and family history.l,2 Due to our and fields to exclude progressive damage. -

Betaxolol Ophthalmic Solution As Alternative Treatment for Patients with Timolol Allergy: a Case Report
Case Report Betaxolol Ophthalmic Solution as Alternative Treatment for Patients with Timolol Allergy: A Case Report Olivia Müllertz 1,*, Jette Jacobsen 2, Jacob P. Thyssen 3, Anna Horwitz 1,4 and Miriam Kolko 1,4,* 1 Department of Drug Design and Pharmacology, University of Copenhagen, 2100 Copenhagen, Denmark; [email protected] 2 Department of Pharmacy, University of Copenhagen, 2100 Copenhagen, Denmark; [email protected] 3 Department of Dermatology and Allergy, Herlev-Gentofte Hospital, 2900 Hellerup, Denmark; [email protected] 4 Department of Ophthalmology, Copenhagen University Hospital, Rigshospitalet, 2600 Glostrup, Denmark * Correspondence: [email protected] (O.M.); [email protected] (M.K.) Received: 24 March 2020; Accepted: 25 June 2020; Published: 30 July 2020 Abstract: Background: To establish if an allergy towards all β-blockers, as a group, should be assumed, if an allergic reaction is observed while using one specific β-blocking agent. Case presentation: The non-selective β-blocker timolol caused a severe allergic ocular reaction in a non-atopic patient with advanced primary open-angle glaucoma. Results: A patch test confirmed timolol allergy. No allergic reaction to other anti-glaucomatous topical drugs was observed, and treatment with the selective β-blocker betaxolol was successfully initiated. Conclusion: Allergy to the non-selective β-blocker timolol does not necessarily predict allergy to the selective β-blocker betaxolol, and betaxolol should therefore not be excluded as an alternative treatment. Keywords: betaxolol; timolol; β-blocker; allergy 1. Introduction Drug allergies are a class of unpredictable adverse reactions that are not related to the pharmacological effect of a drug, and can occur at subtherapeutic doses. -

Betaxolol Hydrochloride
Contains Nonbinding Recommendations Draft Guidance on Betaxolol Hydrochloride This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Betaxolol hydrochloride Dosage Form; Route: Suspension/drops; ophthalmic Strength: EQ 0.25 % Base Recommended Studies: One study Type of study: Bioequivalence (BE) study with clinical endpoint Design: Randomized (1:1), double-masked, parallel, two-arm in vivo Strength: EQ 0.25 % Base Subjects: Males and females with chronic open-angle glaucoma or ocular hypertension in both eyes Additional comments: Specific recommendations are provided below ______________________________________________________________________________ Analytes to measure (in appropriate biological fluid): Not applicable Bioequivalence based on (95% CI): Clinical endpoint Additional comments regarding the BE study with clinical endpoint: 1. The Office of Generic Drugs (OGD) recommends conducting a BE study with a clinical endpoint in the treatment of open-angle glaucoma and ocular hypertension comparing the test product versus the reference listed drug (RLD), each applied as one drop in both eyes two times daily at approximately 8:00 a.m., and 8:00 p.m. for 42 days (6 weeks). 2. Inclusion criteria (the sponsor may add additional criteria): a. Male or nonpregnant females aged at least 18 years with chronic open-angle glaucoma or ocular hypertension in both eyes b. -

Drug Class Review Ophthalmic Beta-Adrenergic Antagonists
Drug Class Review Ophthalmic Beta-Adrenergic Antagonists 52:40.08 Beta-Adrenergic Blocking Agents Betaxolol (Betoptic®) Carteolol (Ocupress®) Levobunolol (Betagan®) Metipranolol (Optipranolol®) Timolol (Betimol®, Timoptic®, others) Timolol/Brimonidine (Combigan®) Timolol/Dorzolamide (Cosopt®; Cosopt PF®) Final Report November 2015 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Carin Steinvoort, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright © 2015 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Executive Summary ......................................................................................................................... 3 Introduction .................................................................................................................................... 5 Table 1. Glaucoma Therapies ................................................................................................. 6 Table 2. Summary of Agents .................................................................................................. 7 Disease Overview ...................................................................................................................... 10 Table 3. Summary of Current Clinical Practice Guidelines .................................................. 11 Pharmacology .............................................................................................................................. -

Β Blockers Timolol Betaxolol Carteolol Ocular Hypotensives: List The
1 Q Ocular Hypotensives: List the common agents blockers Timolol Betaxolol Carteolol 2 A Ocular Hypotensives: List the common agents blockers Timolol Betaxolol Carteolol 3 Q Ocular Hypotensives: List the common agents blockers Timolol Betaxolol Carteolol Prostaglandin analogues Latanaprost Travaprost The ‘big three’ FDA-approved PGA that dominate the American market Bimataprost (Tafluprost) An FDA-approved PGA, much less well-known than the big three (Latanaprostene bunod) A PGA ‘combo drug’ 4 A Ocular Hypotensives: List the common agents blockers Timolol Betaxolol Carteolol Prostaglandin analogues Latanaprost Travaprost The ‘big three’ FDA-approved PGA that dominate the American market Bimataprost (Tafluprost) agonistAn FDA-approved PGA, much less well-known than the big three (Latanaprostene bunod) A PGA ‘combo drug’ 5 Q Ocular Hypotensives: List the common agents blockers Timolol Betaxolol Carteolol Prostaglandin analogues Latanaprost Travaprost The three FDA-approved PGA that dominate the American market Bimataprost What is the brand name of tafluprost? (Tafluprost) agonistAn FDA-approved PGA, much less well-known than the big three Zioptan (and that’s all we’ll have to say about it) (Latanaprostene bunod) A PGA combo drug 6 A Ocular Hypotensives: List the common agents blockers Timolol Betaxolol Carteolol Prostaglandin analogues Latanaprost Travaprost The three FDA-approved PGA that dominate the American market Bimataprost What is the brand name of tafluprost? (Tafluprost) agonistAn -

Drugs That May Be Used Based on Licensure Designation Page 1 of 7 Updated February 2021 by the Pennsylvania State Board of Optometry
Drugs Which May Be Used Based on Licensure Designation Approved Drugs a.) Administration and prescription of pharmaceutical agents for therapeutic purposes. Optometrists who are certified to prescribe and administer pharmaceutical agents for therapeutic purposes under section 4.1 of the Optometric Practice and Licensure Act (63 P. S. § 244.4a), may prescribe and administer the drugs listed in subsections (c)(1) – (11) below in their practice of optometry. See also 49 Pa. Code § 23.202 for the application procedure for optometrists to administer and prescribe pharmaceutical agents for therapeutic purposes. b.) Administration and prescription of pharmaceutical agents to treat glaucoma. Optometrists who are certified to prescribe and administer pharmaceutical agents to treat glaucoma under section 4.2 of the Optometric Practice and Licensure Act (63 P.S. § 244.4b), may prescribe and administer the drugs listed in subsection (c)(12) below in their practice of optometry. See also 49 Pa. Code § 23.205 for the application procedure for optometrists to administer and prescribe pharmaceutical agents to treat glaucoma. c.) DEA registration reminder. Licensees who hold, or plan to obtain, a DEA registration are reminded to review and comply with PDMP standards. d.) Allowable pharmaceutical products. Optometrists may prescribe and administer the following pharmaceutical products or the A-rated generic therapeutically equivalent drug: (1) Topical Anesthetics (i) benoxinate (ii) lidocaine (iii) proparacaine (iv) tetracaine (2) Topical Ocular Lubricants -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P)