Archidendron Bubalinum) Fruit Peel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development of Coffee Cultivation in the Traditional Agroforestry Of
BIODIVERSITAS ISSN: 1412-033X Volume 20, Number 10, October 2019 E-ISSN: 2085-4722 Pages: 2958-2969 DOI: 10.13057/biodiv/d201026 The development of coffee cultivation in the traditional agroforestry of mixed-garden (dukuh lembur) to provide social-economic benefit for the Outer Baduy Community, South Banten, Indonesia BUDIWATI S. ISKANDAR1, JOHAN ISKANDAR2, BUDI IRAWAN2, SUROSO3, RUHYAT PARTASASMITA2,♥ 1Department of Anthropology, Faculty of Social and Political Science, Universitas Padjadjaran. Jl. Raya Bandung-Sumedang Km 21, Kampus Jatinangor, Sumedang 45363, West Java, Indonesia 2Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran. Jl. Raya Bandung-Sumedang Km 21, Jatinangor, Sumedang 45363, West Java, Indonesia. Tel.: +62-22-7797712. email: [email protected]; [email protected] 3Program of Biology, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Kampus Jatinangor, Sumedang 45363, West Java. Indonesia Manuscript received: 17 August 2019. Revision accepted: 16 September 2019. Abstract. Iskandar BS, Iskandar J, Irawan I, Suroso, Partasasmita R. 2019. The development of coffee cultivation in the traditional agroforestry of mixed-garden (dukuh lembur) to provide social-economic benefit for the Outer Baduy Community, South Banten, Indonesia. Biodiversitas 20: 2958-2969. The Baduy community who resides in the Village of Kanekes, the Sub-district of Leuwidamar, the District of Lebak, South Banten has maintained the Sundanese tradition, particularly in practicing swidden farming (ngahuma). They practice swidden farming based on Traditional Ecological Knowledge (TEK) and belief. According to the Baduy tradition, the commercial plants, including coffee, clove, cacao, teak, and rubber have been prohibited to cultivate in Baduy area. However, because the population has increased rapidly and market economy has intensively penetrated the Baduy area, some commercial plants, including robusta coffee (Coffea canephora Pierre ex A. -

Best Practice Guidelines for the Prevention and Mitigation of Conflict Between Humans and Great Apes Kimberley Hockings and Tatyana Humle
Best Practice Guidelines for the Prevention and Mitigation of Conflict Between Humans and Great Apes Kimberley Hockings and Tatyana Humle Series Editor: E.A. Williamson Occasional Paper of the IUCN Species Survival Commission No. 37 IUCN Founded in 1948, IUCN (International Union for Conservation of Nature) brings together States, government agencies and a diverse range of non-governmental organizations in a unique world partnership: over 1000 members in all, spread across some 160 countries. As a Union, IUCN seeks to influence, encourage and assist societies throughout the world to conserve the integrity and diversity of nature and to ensure that any use of natural resources is equitable and ecologically sustainable. IUCN builds on the strengths of its members, networks and partners to enhance their capacity and to support global alliances to safeguard natural resources at local, regional and global levels. IUCN Species Survival Commission The Species Survival Commission (SSC) is the largest of IUCN’s six volunteer commissions with a global membership of 8,000 experts. SSC advises IUCN and its members on the wide range of technical and scientific aspects of species conservation and is dedicated to securing a future for biodiversity. SSC has significant input into the international agreements dealing with biodiversity conservation. Web: www.iucn.org/themes/ssc IUCN Species Programme The IUCN Species Programme supports the activities of the IUCN Species Survival Commission and individual Specialist Groups, as well as implementing global species conservation initiatives. It is an integral part of the IUCN Secretariat and is managed from IUCN’s international headquarters in Gland, Switzerland. The Species Programme includes a number of technical units covering Wildlife Trade, the Red List, Freshwater Biodiversity Assessments (all located in Cambridge, UK), and the Global Biodiversity Assessment Initiative (located in Washington DC, USA). -

Life Science 8 (2) (2019)
Life Science 8 (2) (2019) Life Science http://journal.unnes.ac.id/sju/index.php/LifeSci Dominansi Jenis-Jenis Tanaman Sayur Introduksi di Pasar Sayuran Kota Bengkulu Wiryono 1) dan Steffanie Nurliana2 1)Jurusan Kehutanan, Fakultas Pertanian, Universitas Bengkulu 2)Jurusan Biologi, Fakultas MIPA Universitas Bengkulu Info Artikel Abstract Diterima: 10 September 2019 Since the beginning of agriculture revolution several thousand years ago, human has distributed food plant Disetujui: 20 Oktober 2019 species far beyond their natural range. The tendency of agriculture practice to plant certain “superior” Dipublikasikan: 25 November species and varieties has led to the homogenization of dominant food plant commodities worldwide. Local 2019 species and varieties are marginalized with the introduction of exotic species, resulting in the decline of Keywords biodiversitas, genetic diversity. The objectives of this study were: 1) to determine the species richness of vegetable plants in ethnobotani, ketahanan pangan. three traditional markets in Bengkulu City, 2) to know the species composition of the vegetable plants in those markets, and 3) to determine the species similarity index among the three markets. Data were gathered by recording all species of vegetable plants in the markets. The data were, then, analyzed to determine the species richness, species composition and species similarity among the three markets. The results showed that 1) the total number of vegetable plant species in the three markets was 50, consisting of 23 families, 2) the introduced species dominated the composition of vegetable plants, and 3) the similarity index among markets were >90%. These results confirm the tendency that food plant species composition is dominated by few species only, and show that the species composition of vegetable plants among markets in Bengkulu city was highly similar Abstrak Sejak dimulainya revolusi pertanian beberapa ribu tahun yang lalu, manusia telah memperluas penyebaran jenis tanaman pangan sampai jauh dari wilayah sebaran aslinya. -

Asian Traditions of Wellness
BACKGROUND PAPER Asian Traditions of Wellness Gerard Bodeker DISCLAIMER This background paper was prepared for the report Asian Development Outlook 2020 Update: Wellness in Worrying Times. It is made available here to communicate the results of the underlying research work with the least possible delay. The manuscript of this paper therefore has not been prepared in accordance with the procedures appropriate to formally-edited texts. The findings, interpretations, and conclusions expressed in this paper do not necessarily reflect the views of the Asian Development Bank (ADB), its Board of Governors, or the governments they represent. The ADB does not guarantee the accuracy of the data included in this document and accepts no responsibility for any consequence of their use. The mention of specific companies or products of manufacturers does not imply that they are endorsed or recommended by ADB in preference to others of a similar nature that are not mentioned. Any designation of or reference to a particular territory or geographic area, or use of the term “country” in this document, is not intended to make any judgments as to the legal or other status of any territory or area. Boundaries, colors, denominations, and other information shown on any map in this document do not imply any judgment on the part of the ADB concerning the legal status of any territory or the endorsement or acceptance of such boundaries. ASIAN TRADITIONS OF WELLNESS Gerard Bodeker, PhD Contents I. INTRODUCTION .............................................................................................................................. -

The Journal of Social Sciences Research ISSN(E): 2411-9458, ISSN(P): 2413-6670 Vol
The Journal of Social Sciences Research ISSN(e): 2411-9458, ISSN(p): 2413-6670 Vol. 6, Issue. 1, pp: 85-96, 2020 Academic Research Publishing URL: https://arpgweb.com/journal/journal/7 Group DOI: https://doi.org/10.32861/jssr.61.85.96 Original Research Open Access Construction of Hoax Circulated in Social Media Nini Ibrahim Fakultas Keguruan dan Ilmu Pendidikan, Universitas Muhammadiyah Prof. DR. HAMKA, Jakarta, Indonesia Fauzi Rahman* Fakultas Bahasa dan Seni, Universitas Indraprasta PGRI, Jakarta, Indonesia Abstract This study aims at explore the construction of hoax circulation discourse that causes anxiety and emotions for individuals and community groups. This study was a qualitative analysis method that produced description data in the form of words, sentences, and ideas about nature, circumstances, symptoms, and motivations that arose from certain objects. Data sources for this research were news circulated online: (1) Artificial eggs from China, (2) A Mysterious lecturer in Yogyakarta, and (3) „Jengkol‟ (Archidendron pauciflorum) is as an anticancer medicine. The study of hoax discourse construction is important to be conducted so that people do not easily believe in news related to sources and the validity that cannot be accounted for. This research found out that hoax created by: 1) using sensational and provocative titles, 2) using visual elements as an attraction, 3) using unpopular scientific diction, 4) sometimes threatening, 5) quoting invalid/credible sources, 6) not only sourced from blogs, but from official sites, but circulated in the readers' column, 7) following the latest issues in the community. Keywords: Fake news; Hoax; Critical discourse analysis; Social media. CC BY: Creative Commons Attribution License 4.0 1. -

Tree Diversity and Its Use by Local Communities in Buol District, Indonesia
Tree diversity and its use by local communities in Buol District, Indonesia Subekti Rahayu, Betha Lusiana, Sacha Amaruzaman, Dienda Citasyari Hendrawan dan Sidiq Pambudi Tree diversity and its use by local communities in Buol District, Indonesia Subekti Rahayu, Betha Lusiana, Sacha Amaruzaman, Dienda Citasyari Putri Hendrawan and Sidiq Pambudi Working paper no. 212 LIMITED CIRCULATION Citation: Rahayu S, Lusiana B, Amaruzaman S, Hendrawan DC, Pambudi S. 2015. Tree diversity and its use in Buol District, Indonesia. Working Paper 212. Bogor, Indonesia: World Agroforestry Centre (ICRAF) Southeast Asia Regional Program. DOI: http://dx.doi.org/10.5716/WP15723.PDF Titles in the Working Paper Series aim to disseminate interim results on agroforestry research and practices and stimulate feedback from the scientific community. Other publication series from the World Agroforestry Centre include: agroforestry perspectives, technical manuals and occasional papers. Published by the World Agroforestry Centre (ICRAF) Southeast Asia Regional Program PO Box 161, Bogor 16001 Indonesia Tel: +62 251 8625415 Fax: +62 251 8625416 Email: [email protected] Website: http://www.worldagroforestry.org/regions/southeast_asia © World Agroforestry Centre 2015 Working Paper no. 212 Disclaimer and copyright The views expressed in this publication are those of the author(s) and not necessarily those of the World Agroforestry Centre. Articles appearing in this publication may be quoted or reproduced without charge, provided the source is acknowledged. All images remain the sole property of their source and may not be used for any purpose without written permission of the source. About the authors Subekti Rahayu is a biodiversity and carbon stock specialist at the World Agroforestry Centre. -

Modos Rurales De Representación Y Gestión De Los Sistemas Agrosilvícolas En La Periferia Del Parque Nacional Kerinci Seblat, Sumatra, Indonesia
DOCUMENTO DE TRABAJO QE PUEILOS Y PLANTAS - NOVIEMBRE DE 1998 Esta colección de documentos de trabajo responde a la doble voluntad de informar y de generar un debate fructífero sobre temas Modos ruralesde fundamentales relacionados con el uso representacióny gestión de sostenible y equitativo de los recursos vegetales. IOS sistemasagrosilvícolas en Puede remitirse todo comentario sobre el la periferia del ParqueNac ional presente documento o cualquier sugerencia Kerinci Seblat,Sumatra, para números futuros Las denominaciones e ilustraciones que figuran en esta publicación no entrañan juicio alguno por parte de la UNESCOacerca del estatuto jurídico de ningún país, territorio, ciudad o región o de sus respectivasautoridades, como tampoco acerca del trazado de sus fronteras o límites. Las opiniones expresadasen este documento habrán de atribuirse exclusivamente al autor, sin que la institución donde éste trabaja ni la UNESCOdeban suscribirlas necesariamente. Señasdel autor: Yildiz Aumeeruddy Laboratoire de Botanique - Université de Montpellier II 163, rue Auguste Broussonnet 34000 Montpellier - Francia Fotografías: Thierry Thomas Ilustración de cubierta: Yildiz Aumeeruddy Publicado en 1998 [a partir del original francés de 19941por la Organización de las Naciones Unidas para la Educación, la Ciencia y la Cultura, UNESCO,7 Place de Fontenoy, 75352 Paris CEDEX 07 SP. Impreso por Publicaciones de la UNESCO sobre papel reciclado sin cloro. Editora de la colección: Alison Semple Diseño y compaginación: Ivette Fabbri Traducción del original inglés al castellano: Oriol Canals Compaginación de la traducción castellana: Eric Frogé Referenciarecomendada: Aumeeruddy, Y. (1998). Modos rurales de representación y gestión de los sistemas agrosilvícolas en la periferia del Parque Nacional Kerinci Seblat, Sumatra, Indonesia. Documentos de trabajo de Pueblosy Plantas, no 3. -

Litter and Soil Carbon Stock in Cultivated and Natural Area of Intergrated Forest for Conservation Education of Wan Abdul Rachman Great Forest Park
Available online at: http://journal.unila.ac.id/index.php/tropicalsoil 171 DOI: 10.5400/jts.2016.21.3.171 J Trop Soils, Vol. 21, No. 3, 2016: 171-178 Litter and Soil Carbon Stock in Cultivated and Natural Area of Intergrated Forest for Conservation Education of Wan Abdul Rachman Great Forest Park Leoni Dellta Ellannia1, Agus Setiawan1 and Ainin Niswati2 1Department of Forestry, Faculty of Agriculture,University of Lampung, 2Department of Soil Science, Faculty of Agriculture,University of Lampung, Jalan Sumantri Brojonegoro No. 1, Bandar Lampung, Indonesia, e-mail: [email protected] Received 29 January 2016/ accepted 10 August 2016 ABSTRACT Intergrated Forest for Conservation Education of Wan Abdul Rachman (IFCE WAR) Great Forest Park is a conservation forest zone which has natural area and cultivated area. The natural area in Wan Abdul Rachman Great Forest Park consists of secondary forest, whereas the cultivated area consists of agroforestry with cacao plants and agroforestry with coffee plants. The different land use in both areas caused the difference in carbon sink specifically in litter and soil. The research was aimed to study the difference of litter and soil carbon stock in natural and cultivated area in IFCE WAR Great Forest Park. The observation plots included in the current study was determined using purposive sampling method. The research was conducted in June until August 2015. Data was analyzed using analysis of variance and continued with honestly significant difference test. The results showed that there was no difference of litter carbon stock in cultivated area and natural area in IFCE WAR Great Forest Park, whereas the soil carbon stock in natural area was higher than that in cultivated area. -

Microwave Assisted Extraction (Mae) of Beta-Sitosterol from Plant Legumes Pod
CORE Metadata, citation and similar papers at core.ac.uk Provided by UMP Institutional Repository MICROWAVE ASSISTED EXTRACTION (MAE) OF BETA-SITOSTEROL FROM PLANT LEGUMES POD NORLAILI BINTI HASHIM MASTERS OF SCIENCE UNIVERSITI MALAYSIA PAHANG SUPERVISOR’s DECLARATION We hereby declare that we have checked this thesis and in our opinion, this thesis is adequate in terms of scope and quality for the award of the degree of Master of Science _______________________________ (Supervisor’s Signature) Full Name : DR. NOORMAZLINAH.BINTI AHMAD Position : SENIOR LECTURER Date : _______________________________ (Co-supervisor’s Signature) Full Name : DR. NURUL AINI BINTI AZMAN Position : SENIOR LECTURER Date : STUDENT’S DECLARATION I hereby declare that the work in this thesis is based on my original work except for quotations and citations which have been duly acknowledged. I also declare that it has not been previously or concurrently submitted for any other degree at Universiti Malaysia Pahang or any other institutions. _______________________________ (Student’s Signature) Full Name : NORLAILI BINTI HASHIM ID Number : MKT16001 Date : MICROWAVE ASSISTED EXTRACTION (MAE) OF BETA-SITOSTEROL FROM PLANT LEGUMES POD NORLAILI BINTI HASHIM Thesis submitted in fulfillment of the requirements for the award of the degree of Master of Science Faculty of Chemical and Natural Resources Engineering UNIVERSITI MALAYSIA PAHANG MARCH 2019 ACKNOWLEDGEMENTS In the Name of Allah, the Beneficent, the Merciful. I would like to thank Allah because always be with me all the time. He always gives me the courage to finish this project especially when I was in trouble. In order to prepare and finish this project report, I was in contact with a lot of people, researchers, academicians, and practitioners. -
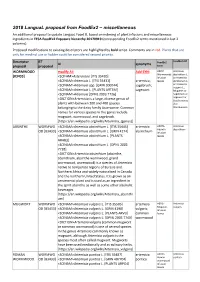
2018 Langual Proposal from Foodex2 – Miscellaneous
2018 LanguaL proposal from FoodEx2 – miscellaneous An additional proposal to update LanguaL Facet B, based on indexing of plant infusions and miscellaneous ingredients in EFSA FoodEx2 Exposure hierarchy 20170919 (corresponding FoodEx2 terms mentioned in last 2 columns). Proposed modifications to existing descriptors are highlighted by bold script. Comments are in red. Plants that are only for medinal use or fodder could be considered second priority. Descriptor BT FoodEx2 FoodEx2 def AI synonyms proposal proposed term WORMWOOD modify AI: Add SYN: A00YY Artemisia Wormwoods absinthium L. [B3433] <SCIFAM>Asteraceae [ITIS 35420] infusion or Artemisia <SCINAM>Artemisia L. [ITIS 35431] artemisia; leaves abrotanum L. <SCINAM>Artemisia spp. [GRIN 300044] sagebrush; or Artemisia vulgaris L., <SCINAM>Artemisia L. [PLANTS ARTEM] sagewort Mugwort or <SCINAM>Artemisia [DPNL 2003 7726] Sagebrush or Artemisia Sagewort or <DICTION> is a large, diverse genus of Southernwoo plants with between 200 and 400 species d or belonging to the daisy family Asteraceae. Common Wormwoods names for various species in the genus include mugwort, wormwood, and sagebrush. [https://en.wikipedia.org/wiki/Artemisia_(genus)] ABSINTHE WORMWO <SCINAM>Artemisia absinthium L. [ITIS 35445] artemisia A007A Artemisia Absinth absinthium OD [B3433] <SCINAM>Artemisia absinthium L. [GRIN 4274] absinthium infusion <SCINAM>Artemisia absinthium L. [PLANTS leaves ARAB3] <SCINAM>Artemisia absinthium L. [DPNL 2003 7728] <DICTION>Artemisia absinthium (absinthe, absinthium, absinthe wormwood, grand wormwood, wormwood) is a species of Artemisia native to temperate regions of Eurasia and Northern Africa and widely naturalized in Canada and the northern United States. It is grown as an ornamental plant and is used as an ingredient in the spirit absinthe as well as some other alcoholic beverages. -

Fruit Diversity for Agrotourism Development in Rawa Bayu, Bayu Village, Songgon, Banyuwangi
Journal of Indonesian Tourism and E-ISSN : 2338-1647 Development Studies http://jitode.ub.ac.id Fruit Diversity for Agrotourism Development in Rawa Bayu, Bayu Village, Songgon, Banyuwangi Ardina Tanjungsari, Azzah Fauziyah Cholis*, Christien Yacobina Riung, Lutvita Erya Rokani Department of Biology, Faculty of Mathematics and Natural Sciences, University of Brawijaya, Malang, Indonesia Abstract Rural area in around Rawa Bayu tourism object has numerous fruit trees species which is possible to be integrated in the development of nature based tourism in Rawa Bayu. The aims of the study were describing the diversity of fruit species, mapping geographic positions of fruits plants grows, and exploring the local perspective about fruit plants species in its relationship with agrotourism development. Research methods consisted of fruit plant species exploration in Rawa Bayu ecotourism and its surrounding area, mapping the coordinates geographic using GPS (Global Positioning System), and implementing semi structured interviews with local respondends. Exploration was done by visiting Rawa Bayu ecotourism and its surrounding area, especially local people’s yard. The fruit plant species was observed morphologically. The geographic position of fruit plant species was mapped using GPS. Semi structured interviews were implemented to 20 respondents, they are consist of locall community, tourist and local tourist in Rawa Bayu. Data was analized descriptively. This research found about 13 fruit plant species grows in Rawa Bayu area. Fruit plant species were potential for rural tourism development program. The most common fruit plant species found were durian (Durio zibethinus), salacca (Salacca zalacca), banana (Musa sp.), guava (Syzygium aqueum), jackfruit (Artocarpus heterophyllus), jengkol (Archidendron pauciflorum), langsat (Lansium domesticum), clove (Syzigium aromaticum), mangosteen (Garcinia mangostana), and avocado (Persea americana). -

1412-033X E-Issn: 2085-4722
ISSN: 1412-033X E-ISSN: 2085-4722 Front cover: Dracocephalum aucheri (PHOTO: KOUROSH KAVOUSI) Published semiannually PRINTED IN INDONESIA ISSN: 1412-033X E-ISSN: 2085-4722 ISSN: 1412-033X E-ISSN: 2085-4722 Evaluation of vegetation types in the West Zagros (Beiranshahr region as a case study), 1-10 in Lorestan Province, Iran ATENA ESLAMI FAROUJI, HAMED KHODAYARI Floristic changes at Khersan Glacier Territory, Alamkuh Mountain, Central Alborz, North 11-15 of Iran KOUROSH KAVOUSI, TAHER NEJADSATARI , YUNES ASRI, HAMID EJTEHADI, RAMEZAN ALI KHAVARI-NEJAD Molecular phylogeny of Acer monspessulanum L. subspecies from Iran inferred using 16-23 the ITS region of nuclear ribosomal DNA HANIF KHADEMI, IRAJ MEHREGAN, MOSTAFA ASSADI, TAHER NEJADSATARI, SHAHIN ZARRE Determination of appropriate grid dimension and sampling plot size for assessment of 24-30 woody species diversity in Zagros Forest, Iran ALI ASGHAR ZOHREVANDI, HASSAN POURBABAEI, REZA AKHAVAN, AMIR ESLAM BONYAD Short communication: 31-35 Algal leaf spot associated with Cephaleuros virescens (Trentepohliales, Ulvophyceae) on Nephelium lappaceum in Thailand ANURAG SUNPAPAO, MUTIARA K. PITALOKA , SIWARET ARIKIT Temporal variability in macroinvertebrates diversity patterns and their relation with 36-43 environmental factors MOHAMMAD HASAN GERAMI, RAHMAN PATIMAR, HOSSEIN NEGARESTAN, HOJJATOLLAH JAFARIAN, MOHAMMAD SEDDIQ MORTAZAVI Status of coastal forests of the Northern Sumatra in 2005 (after 2004’s tsunami 44-54 catastrophe) ONRIZAL, MASHHOR MANSOR The diversity and distribution of Holothuroidea in shallow waters of Baluran National 55-60 Park, Indonesia ARIF MOHAMMAD SIDDIQ, TRI ATMOWIDI, IBNUL QAYIM Ethnobotany of Canarium plant species used by Tobelo Dalam (Togutil) ethnic 61-69 community of Halmahera Island, Indonesia M. NASIR TAMALENE, MIMIEN HENIE IRAWATI AL MUHDHAR, ENDANG SUARSINI, FATCHUR RAHMAN, SAID HASAN Dominance and diversity studies of tree species in lesser Himalayan forest of 70-77 Uttarakhand, India A.S.