דוקטור לפילוסופיה Doctor of Philosophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DSU Music Newsletter
Delaware State University Music Department Spring 2018 Music Department Schedule Tues., Mar. 20, 11am: Music Performance Seminar (Theater) Volume 2: Issue 1 Fall 2018 Tues., Mar. 27, 11am: Music Performance Seminar (Theater) Concert choir to perform with Philadelphia orchestra Friday, April 6, 7:00 PM: Junior Recital; Devin Davis, Tenor, Anyre’ Frazier, Alto, On March 28, 29, and 30 of 2019, Tommia Proctor, Soprano (Dover Presbyterian Church) the Delaware State University Concert Choir under the direction of Saturday, April 7, 5:00 PM: Senior Capstone Recital; William Wicks, Tenor (Dover Presbyterian Church) Dr. Lloyd Mallory, Jr. will once again be joining the Philadelphia Sunday, April 8, 4:00 PM: Orchestra. The choir will be Senior Capstone Recital; Michele Justice, Soprano (Dover Presbyterian Church) performing the world premiere of Healing Tones, by the Orchestra’s Tuesday, April 10, 11:00 AM: Percussion Studio Performance Seminar (EH Theater) composer-in-residence Hannibal Lokumbe. In November of 2015 the Sunday, April 15, 4:00 PM: Delaware State University Choir Senior Capstone Recital; Marquita Richardson, Soprano (Dover Presbyterian Church) joined the Philadelphia Orchestra to Tuesday, April 17, 11:00 AM: DSU – A place where dreams begin perform the world premiere of Guest Speaker, Dr. Adrian Barnes, Rowan University (Music Hannibal’s One Land, One River, One Education/Bands) (EH 138) People. About the performance, the Friday, April 20, 12:30 PM: Philadelphia Inquirer said “The massed voices of the Delaware State University Choir, the Lincoln Honors Day, Honors Recital (EH Theater) University Concert Choir, and Morgan State University Choir sang with spirit, accuracy and, near- Friday, April 20, 7:00 PM: More inside! Pg. -

The Origins, Evolution and Decline of the Khojki Script
The origins, evolution and decline of the Khojki script Juan Bruce The origins, evolution and decline of the Khojki script Juan Bruce Dissertation submitted in partial fulfilment of the requirements for the Master of Arts in Typeface Design, University of Reading, 2015. 5 Abstract The Khojki script is an Indian script whose origins are in Sindh (now southern Pakistan), a region that has witnessed the conflict between Islam and Hinduism for more than 1,200 years. After the gradual occupation of the region by Muslims from the 8th century onwards, the region underwent significant cultural changes. This dissertation reviews the history of the script and the different uses that it took on among the Khoja people since Muslim missionaries began their activities in Sindh communities in the 14th century. It questions the origins of the Khojas and exposes the impact that their transition from a Hindu merchant caste to a broader Muslim community had on the development of the script. During this process of transformation, a rich and complex creed, known as Satpanth, resulted from the blend of these cultures. The study also considers the roots of the Khojki writing system, especially the modernization that the script went through in order to suit more sophisticated means of expression. As a result, through recording the religious Satpanth literature, Khojki evolved and left behind its mercantile features, insufficient for this purpose. Through comparative analysis of printed Khojki texts, this dissertation examines the use of the script in Bombay at the beginning of the 20th century in the shape of Khoja Ismaili literature. -
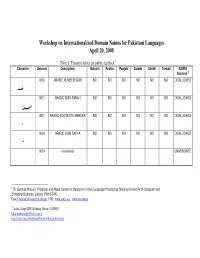
Workshop on Internationalized Domain Names for Pakistani Languages April 20, 2008
Workshop on Internationalized Domain Names for Pakistani Languages April 20, 2008 Table 1: Tentative tables for public feedback 1 Character Unicode Description Balochi Pashto Punjabi Saraiki Sindhi Torwali ASIWG Decision 2 0600 ARABIC NUMBER SIGN NO NO NO NO NO NO DISALLOWED 0601 ARABIC SIGN SANAH NO NO NO NO NO NO DISALLOWED 0601 ARABIC FOOTNOTE MARKER NO NO NO NO NO NO DISALLOWED 0603 ARABIC SIGN SAFHA NO NO NO NO NO NO DISALLOWED 0604 <reserved> UNASSIGNED 1 Dr. Sarmad Hussain, Professor and Head, Center for Research in Urdu Language Processing, National University of Computer and Emerging Sciences, Lahore, PAKISTAN. Email: [email protected] , URL: www.crulp.org , www.nu.edu.pk 2 Arabic Script IDN Working Group (ASIWG) [email protected] http://lists.irnic.ir/mailman/listinfo/idna-arabicscript 0605 <reserved> UNASSIGNED 0606 ARABIC-INDIC CUBE ROOT NO NO NO NO NO NO DISALLOWED 0607 ARABIC-INDIC FOURTH NO NO NO NO NO NO DISALLOWED ROOT 0608 ARABIC RAY NO NO NO NO NO NO DISALLOWED 0609 ARABIC-INDIC PER MILLE NO NO NO NO NO NO DISALLOWED SIGN 060A ARABIC-INDIC PER TEN NO NO NO NO NO NO DISALLOWED THOUSAND SIGN 060B AFGHANI SIGN NO NO NO NO NO NO DISALLOWED 060C ARABIC COMMA NO NO NO NO NO NO DISALLOWED 060D ARABIC DATE SEPARATOR NO NO NO NO NO NO DISALLOWED ؍ 060E ARABIC POETIC VERSE SIGN NO NO NO NO NO NO DISALLOWED ؎ 060F ARABIC SIGN MISRA NO NO NO NO NO NO DISALLOWED ؏ 0610 ARABIC SIGN SALLALLAHOU PENDING PENDING PENDING PENDING PENDING PENDING PVALID ALAYHE WASSALLAM 0611 ARABIC SIGN ALAYHE PENDING PENDING -

Unicode Alphabets for L ATEX
Unicode Alphabets for LATEX Specimen Mikkel Eide Eriksen March 11, 2020 2 Contents MUFI 5 SIL 21 TITUS 29 UNZ 117 3 4 CONTENTS MUFI Using the font PalemonasMUFI(0) from http://mufi.info/. Code MUFI Point Glyph Entity Name Unicode Name E262 � OEligogon LATIN CAPITAL LIGATURE OE WITH OGONEK E268 � Pdblac LATIN CAPITAL LETTER P WITH DOUBLE ACUTE E34E � Vvertline LATIN CAPITAL LETTER V WITH VERTICAL LINE ABOVE E662 � oeligogon LATIN SMALL LIGATURE OE WITH OGONEK E668 � pdblac LATIN SMALL LETTER P WITH DOUBLE ACUTE E74F � vvertline LATIN SMALL LETTER V WITH VERTICAL LINE ABOVE E8A1 � idblstrok LATIN SMALL LETTER I WITH TWO STROKES E8A2 � jdblstrok LATIN SMALL LETTER J WITH TWO STROKES E8A3 � autem LATIN ABBREVIATION SIGN AUTEM E8BB � vslashura LATIN SMALL LETTER V WITH SHORT SLASH ABOVE RIGHT E8BC � vslashuradbl LATIN SMALL LETTER V WITH TWO SHORT SLASHES ABOVE RIGHT E8C1 � thornrarmlig LATIN SMALL LETTER THORN LIGATED WITH ARM OF LATIN SMALL LETTER R E8C2 � Hrarmlig LATIN CAPITAL LETTER H LIGATED WITH ARM OF LATIN SMALL LETTER R E8C3 � hrarmlig LATIN SMALL LETTER H LIGATED WITH ARM OF LATIN SMALL LETTER R E8C5 � krarmlig LATIN SMALL LETTER K LIGATED WITH ARM OF LATIN SMALL LETTER R E8C6 UU UUlig LATIN CAPITAL LIGATURE UU E8C7 uu uulig LATIN SMALL LIGATURE UU E8C8 UE UElig LATIN CAPITAL LIGATURE UE E8C9 ue uelig LATIN SMALL LIGATURE UE E8CE � xslashlradbl LATIN SMALL LETTER X WITH TWO SHORT SLASHES BELOW RIGHT E8D1 æ̊ aeligring LATIN SMALL LETTER AE WITH RING ABOVE E8D3 ǽ̨ aeligogonacute LATIN SMALL LETTER AE WITH OGONEK AND ACUTE 5 6 CONTENTS -

Makeyourfont-Abdulkerim.Pdf
اﺻﻨ َﻊ َﺧ ﱠﻄ ﻚ ﺑﻨ َ ْﻔ ﺴ ِﻚ ﺗﺄﻟﻴﻒ ُﻣ َـﺤ ﱠﻤـﺪ َﳛ َﻴﻰ َﻋ ْﺒﺪ َاﻟﻜ ِﺮ ْﻳﻢ أﺧﺼﺎﺋﻲ ﺑﺮاﻣﺞ آﻟﻴﺔ وﺣﺎﺳﺒﺎت ﺑﴩﻛﺔ اﻟﺼﻔﻮة ﻟﻠﱪﳎﻴﺎت − اﻟﻘﺎﻫﺮة − ﻣﴫ ﻣﺮاﺟﻌﺔ وﺗﺪﻗﻴﻖ وﺗﺮﺗﻴﺐ َﻳ َﻌﲆ َﻗ َﺤﻄﺎن ﱡاﻟﺪ ْو ِر ّي – – ﻛﻠﻴﺔ اﻟﺪراﺳﺎت اﻟﻔﻘﻬﻴﺔ واﻟﻘﺎﻧﻮﻧﻴﺔ ﺟﺎﻣﻌﺔ آل اﻟﺒﻴﺖ اﻷُردن א اﺳﻢ اﻟﻜﺘﺎب: اﺻﻨﻊ ﺧﻄﻚ ﺑﻨﻔﺴﻚ. اﳌﺆﻟﻒ: ُﻣ َـﺤ ﱠﻤـﺪ َﳛ َﻴﻰ َﻋ ْﺒﺪ َاﻟﻜ ِﺮ ْﻳﻢ. ﻣﺮاﺟﻌﺔ وﺗﺪﻗﻴﻖ وﺗﺮﺗﻴﺐ: َﻳ َﻌﲆ َﻗ َﺤﻄﺎن ﱡاﻟﺪ ْو ِر ّي . ﺗﺼﻤﻴﻢ اﻟﻐﻼف: َﻋ ْﺒﺪ اﷲ َﻋ ِﺎرف. اﻟﻤﺆﻟﻒ.... DOS C++ C#Visual Basic Visual Basic.net ًوأﺧـــﲑا: ﻟﻼﺳﺘﻔﺴﺎر ﻳﺮﺟﻰ اﻟﺘﻮاﺻﻞ ﻋﲆ: [email protected] +20188913719 ﺷﻜﺮ ﺧﺎص إﱃ اﻷخ اﻟﻜﺮﻳﻢ: َﻳ َﻌﲆ َﻗ َﺤﻄ ﺎن ﱡاﻟﺪ ْو ِر ّي . http://www.graphics4arab.com http://indesignyat.webatu.com http://zakdesign.net http://www.rashedy.com أﻣﺎ ﺑﻌﺪ: اﺻﻨﻊ ﺧﻄﻚ ﺑﻨﻔﺴﻚ واﷲ اﳌﻮﻓﻖ @@@éjn× áíŠØÛa@†jÇ@óî°@†àª اﺻﻨﻊ ﺧﻄﻚ ﺑﻨﻔﺴﻚ ﺗﻌﺮﻳﻒ اﳋﻂ اﳊﺎﺳﻮﰊ [Font File]: E١F Font Font Font File Encoding Character Mapping Traditional Arabic E١F اﺻﻨﻊ ﺧﻄﻚ -
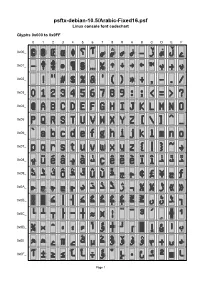
Psftx-Debian-10.5/Arabic-Fixed16.Psf Linux Console Font Codechart
psftx-debian-10.5/Arabic-Fixed16.psf Linux console font codechart Glyphs 0x000 to 0x0FF 0 1 2 3 4 5 6 7 8 9 A B C D E F 0x00_ 0x01_ 0x02_ 0x03_ 0x04_ 0x05_ 0x06_ 0x07_ 0x08_ 0x09_ 0x0A_ 0x0B_ 0x0C_ 0x0D_ 0x0E_ 0x0F_ Page 1 Glyphs 0x100 to 0x1FF 0 1 2 3 4 5 6 7 8 9 A B C D E F 0x10_ 0x11_ 0x12_ 0x13_ 0x14_ 0x15_ 0x16_ 0x17_ 0x18_ 0x19_ 0x1A_ 0x1B_ 0x1C_ 0x1D_ 0x1E_ 0x1F_ Page 2 Font information 0x016 U+2026 HORIZONTAL ELLIPSIS Filename: psftx-debian-10.5/Arabic-Fixed16.ps 0x017 U+2030 PER MILLE SIGN f PSF version: 1 0x018 U+2191 UPWARDS ARROW, U+25B2 BLACK UP-POINTING Glyph size: 8 × 16 pixels TRIANGLE, Glyph count: 512 U+25B4 BLACK UP-POINTING Unicode font: Yes (mapping table present) SMALL TRIANGLE 0x019 U+2193 DOWNWARDS ARROW, Unicode mappings U+25BC BLACK DOWN-POINTING 0x000 U+00A9 COPYRIGHT SIGN TRIANGLE, U+25BE BLACK DOWN-POINTING 0x001 U+00AE REGISTERED SIGN SMALL TRIANGLE 0x002 U+0152 LATIN CAPITAL LIGATURE 0x01A U+2192 RIGHTWARDS ARROW, OE U+25B6 BLACK RIGHT-POINTING TRIANGLE, 0x003 U+0153 LATIN SMALL LIGATURE OE U+25B8 BLACK RIGHT-POINTING 0x004 U+2666 BLACK DIAMOND SUIT, SMALL TRIANGLE U+25C8 WHITE DIAMOND 0x01B U+2190 LEFTWARDS ARROW, CONTAINING BLACK SMALL U+25C0 BLACK LEFT-POINTING DIAMOND, TRIANGLE, U+FFFD REPLACEMENT U+25C2 BLACK LEFT-POINTING CHARACTER SMALL TRIANGLE 0x005 U+061F ARABIC QUESTION MARK 0x01C U+2122 TRADE MARK SIGN 0x006 U+0622 ARABIC LETTER ALEF WITH 0x01D U+FB53 ARABIC LETTER BEEH MADDA ABOVE FINAL FORM 0x007 U+0633 ARABIC LETTER SEEN 0x01E U+FB55 ARABIC LETTER BEEH MEDIAL FORM 0x008 U+0634 ARABIC LETTER -

Proposal to Encode Productive Arabic-Script Modifier Marks 2
Proposal to encode productive Arabic-script modifier marks Date: May 16, 2003 Author: Jonathan Kew, SIL International Kamal Mansour, Agfa Monotype Mark Davis, IBM Address: Horsleys Green Agfa Monotype High Wycombe 425 Sherman Ave Bucks HP14 3XL Palo Alto, CA 94306 England USA Tel: +44 (1494) 682306 +1 (650) 321-4102 Email: [email protected] [email protected] [email protected] A. Administrative 1. Title Proposal to encode productive Arabic-script modifier marks 2. Requester’s name SIL International (contacts: Jonathan Kew, Peter Constable); Agfa Monotype (contact: Kamal Mansour) 3. Requester type Expert contribution 4. Submission date May 16, 2003 5. Requester’s reference 6a. Completion This is a complete proposal. 6b. More information to be provided? Only as required for clarification. B. Technical — General 1a. New script? Name? No 1b. Addition of characters to existing block? Yes — Arabic Name? 2. Number of characters in proposal 23 3. Proposed category A 4. Proposed level of implementation and 2 (includes combining marks) rationale 5a. Character names included in proposal? Yes 5b. Character names in accordance with Yes guidelines? 5c. Character shapes reviewable? Yes 6a. Who will provide computerized font? Jonathan Kew, SIL International 6b. Font currently available? Yes 6c. Font format? TrueType 7a. Are references (to other character sets, See the documents cited under References. dictionaries, descriptive texts, etc.) provided? 7b. Are published examples (such as samples See the documents cited under References. from newspapers, magazines, or other sources) of use of proposed characters attached? 8. Does the proposal address other aspects of Yes, suggested character properties and collation character data processing? weights are included. -

International Terrorism >'; / ~'
If you have issues viewing or accessing this file contact us at NCJRS.gov. TER~\PRISTIC AC.TIVITY-) International Terrorism >'; / ~' BEFORE THE ,~'!, : "'< SUBCOMMITTEE TO INVESTIGATE THE AD})!IINISTRATION OF THE INTERNAL SECURITY AOT AND OTHER INTERNAL SECURITY LAWS " i 0]' THE J COMMITTEE ON THE JUDICIARY UNITED STATES SENATE NINETY-FOURTH CONGRESS FIRST SESSION t l 1 '-..::) PART 4 " r I MAY 14, 1975 - t "" \ Printed for the use of the Committee on the Judiciary (S J. f u.s. GOVERNMENT PRINTING OFFICE If) WASHINGTON : 1975 ale by the Supurlntendent or Documents, U.S. Government I'rluting Office '~1.- Washington, D.C. 2(H02· Prico $1.20 ~,¢ ",.IJ ----------,:!'-.------------.pe:-------------------~----------....,.---...,-------------- -----~ ---~ ----~- COMMITTEE;ON THE JUDICIARX ~ .. ~ JA1v1ES O. EASTLAND, Mississippi, Chairman JOHN,L.lIfCOLELLAN, Arknnsus ROMAN L. HRUSKA,NebrasKa PlIILIP A.lIART, Michigan :Ej:IRAM L. FONo-, lIawnll EDWARD l\I. KENNEDY, lIfassachnsetts lIUOli SCOTT, Pennsylvania BIROliBAYlI,lndlone., . , STROIlITllVRMO))/D, South, (jarollnn QUENTIN N. B URDWK, North Dakotil CHARLES .1ireO. ¥A'I'HrAS, ,JR.,; 1\1'nt'Yland ROBERT O. BYRD, West Virginia WILLIAi\f L. SCQTT, Vlrglnlii CONTENTS JOliNI'. TUNNEY, California . Testimony of: JAMES ABOUREZK, SOUt11 D.~kota Mr.London, Brian Crozier,England director ___________________________________________ of the Institute for the Study of Conflict, _ Page 179 Hon. Robert A. Feurey) Special Assistant to the Secretary of l:ltate, Coordinator for Combatting Terrorism, Department of State, SUBCOMMITTEE To INVESTIGATE THE Am.iulISTRATION OF THE INTERN,\L accompanied by lVl!:. Louis Fields and ;dr. John N. Gatch, Deputy __ 212 Appendix ________________ '________________ ~ _______________________ _ SECURa'y ACT ANn OTHE:J;li INT,E::aNA;L SECURITY LAWS 253 JA?>ms o. -

LGR-4 Code Table for Non-CJK Code Points
LGR-4 Code Table for non-CJK code points Integration Panel Date: 2020-06-29 This document shows the repertoire for LGR-4 code points not part of the CJK blocks presented in the form of marked up tables in a format similar to that used for character code charts in the Unicode Standard. Code cells with background color: - Green: code points that are part of the LGR, including all members of code point sequences. - Pink: code points that are not PVALID in IDNA2008 [RFC5892] [IDNAREG]. - White: PVALID code points which are excluded from the Root Zone in a generic fashion (digits, hyphen), or by being excluded from the Maximum Starting Repertoire [MSR-4]. - Lavender: PVALID code points not included in the LGR as results of decisions during the development of the LGR. Unicode blocks that contain no repertoire of the LGR are suppressed. In these code tables, code points are listed as part of the merged LGR (green) even of the LGR does not list the code point by itself, but only defines a code point sequence containing the code point. Code points that are marked “out-of-repertoire” by a reflexive variant mapping of type “out-of-repertoire- var” in any element LGR are shown as part of the merged LGR only if they occur in at least one element LGR without such mapping. [IDNAREG] IANA Registry: "IDNA Parameters". For Unicode 6.3 available at: http://www.iana.org/assignments/idna-tables-6.3.0/idna-tables-6.3.0.xml. Visited 2013-11-20 [MSR-4] Integration Panel, "Maximal Starting Repertoire — MSR-4 Overview and Rationale", 25 January 2019 https://www.icann.org/en/system/files/files/msr-4-overview-25jan19-en.pdf. -

MSR-4: Annotated Repertoire Tables, Non-CJK
Maximal Starting Repertoire - MSR-4 Annotated Repertoire Tables, Non-CJK Integration Panel Date: 2019-01-25 How to read this file: This file shows all non-CJK characters that are included in the MSR-4 with a yellow background. The set of these code points matches the repertoire specified in the XML format of the MSR. Where present, annotations on individual code points indicate some or all of the languages a code point is used for. This file lists only those Unicode blocks containing non-CJK code points included in the MSR. Code points listed in this document, which are PVALID in IDNA2008 but excluded from the MSR for various reasons are shown with pinkish annotations indicating the primary rationale for excluding the code points, together with other information about usage background, where present. Code points shown with a white background are not PVALID in IDNA2008. Repertoire corresponding to the CJK Unified Ideographs: Main (4E00-9FFF), Extension-A (3400-4DBF), Extension B (20000- 2A6DF), and Hangul Syllables (AC00-D7A3) are included in separate files. For links to these files see "Maximal Starting Repertoire - MSR-4: Overview and Rationale". How the repertoire was chosen: This file only provides a brief categorization of code points that are PVALID in IDNA2008 but excluded from the MSR. For a complete discussion of the principles and guidelines followed by the Integration Panel in creating the MSR, as well as links to the other files, please see “Maximal Starting Repertoire - MSR-4: Overview and Rationale”. Brief description of exclusion -

A Study on Collation of Languages from Developing Asia
A Study on Collation of Languages from Developing Asia Sarmad Hussain Nadir Durrani Center for Research in Urdu Language Processing National University of Computer and Emerging Sciences www.nu.edu.pk www.idrc.ca Published by Center for Research in Urdu Language Processing National University of Computer and Emerging Sciences Lahore, Pakistan Copyrights © International Development Research Center, Canada Printed by Walayatsons, Pakistan ISBN: 978-969-8961-03-9 This work was carried out with the aid of a grant from the International Development Research Centre (IDRC), Ottawa, Canada, administered through the Centre for Research in Urdu Language Processing (CRULP), National University of Computer and Emerging Sciences (NUCES), Pakistan. ii Preface Defining collation, or what is normally termed as alphabetical order or less frequently as lexicographic order, is one of the first few requirements for enabling computing in any language, second only to encoding, keyboard and fonts. It is because of this critical dependence of computing on collation that its definition is included within the locale of a language. Collation of all written languages are defined in their dictionaries, developed over centuries, and are thus very representative of cultural tradition. However, though it is well understood in these cultures, it is not always thoroughly documented or well understood in the context of existing character encodings, especially the Unicode. Collation is a complex phenomenon, dependent on three factors: script, language and encoding. These factors interact in a complicated fashion to uniquely define the collation sequence for each language. This volume aims to address the complex algorithms needed for sorting out the words in sequence for a subset of the languages. -

Proposal for Arabic Script Root Zone LGR
Proposal for Arabic Script Root Zone LGR LGR Version: 1 Authors: Task Force on Arabic Script IDN (TF-AIDN) https://community.icann.org/display/MES/TF-AIDN+Work+Space Date: 18 November 2015 Document Version: 3.4 Contents General Information ..................................................................................................................................... 2 1 Script and Languages Covered .............................................................................................................. 2 2 Process Undertaken for Developing the Proposal ................................................................................ 4 2.1 Team diversity and process .......................................................................................................... 4 2.2 Analysis of code point repertoire.................................................................................................. 6 2.3 Analysis of code point variants ..................................................................................................... 8 3 Code Point Repertoire......................................................................................................................... 10 3.1 Summary of code point repertoire included and excluded ........................................................ 10 3.2 Code point repertoire included .................................................................................................. 12 4 Final Recommendation of Variants for Top Level Domains (TLDs) ....................................................